SARS-CoV-2, the causative agent of the ongoing coronavirus disease (COVID-19), emerged in late 2019 and has resulted in unprecedented harm to human health and the global economy.
It is known that the viral envelope is decorated with glycosylated spike, which is a large trimeric type I fusion protein. Since most neutralizing antibodies appear to target this spike, it quickly turned into the primary attention focus during the development of non-classical vaccines.
The aforementioned protein has at least two fundamental functions; first, it attaches to the angiotensin-converting enzyme 2 (ACE2) receptor with its receptor-binding domain (RBD) and then acts as a machine to fuse the viral and host cell membranes in order to allow the entry of the viral genome.
This process is not yet fully understood; nonetheless, adequate grasp of spike structure and ensuing conformational changes is pivotal to precisely define the anatomy of the target – central not only for neutralizing antibodies, but also novel or repurposed chemical compounds.
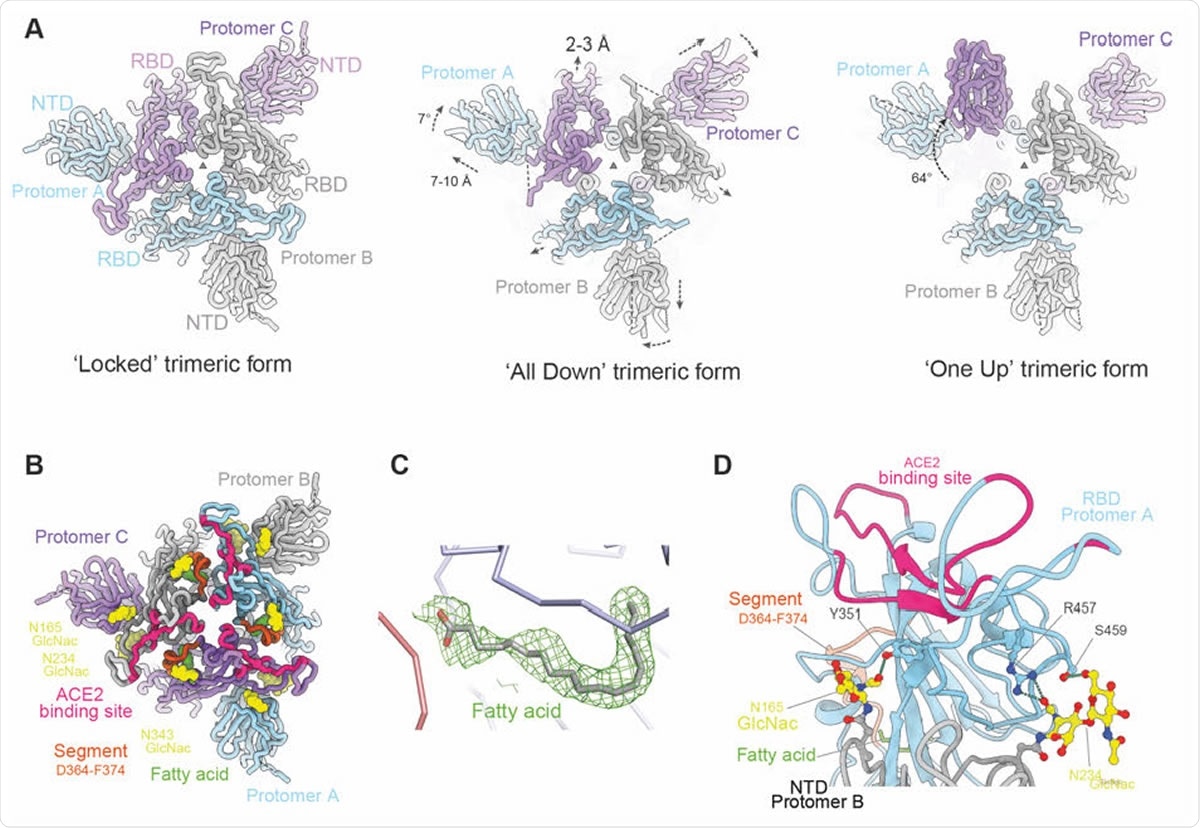
Overall structure of locked trimeric structure of SARS-CoV-2 Spike. (A) Comparison of the lipid bound Spike with the previously observed RBD ‘all down’ (PDB ID, 6VXX) and ‘one up’ Spike structures (PDB ID, 6Z97). The protomers are coloured in pale blue, grey and purple respectively. Relative rotation and translation of the NTD and RBD are indicated. (B) Top view of the RBD and NTD domains of the ‘locked’ Spike showing the relative positions of the bound fatty acids (green surfaces), the ACE2 binding sites are shown in red, α2 helices that have largest movement in brown, glycans at N165 and N234 of the NTD and N343 of RBD as yellow surfaces. (C) Density of the bound fatty acid. (D) Closeup of the interactions between glycans (yellow) on N165 and N234 of protomer B NTD (grey) and protomer A RBD (cyan).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Recognizing the putative receptor
It is still puzzling how the spike interrogates its surroundings for receptors while maintaining the integrity of the prefusion state. Nonetheless, the accepted model is that RBDs stochastically flip up to reveal the ACE2 binding site transiently.
More specifically, based on 'up' and 'down' positions of RBD, spike protein can be in a receptor-accessible open state or receptor-inaccessible closed state, respectively. However, recent observation has shown that purified wild-type spike ectodomain exists as a mixture of a hitherto undescribed 'locked' (all RBDs down) prefusion state and a postfusion state.
A group of scientists from the University of Oxford reported a high-resolution cryogenic electron microscopy structure of SARS-CoV-spike ectodomain, revealing a firmly packed arrangement of the spike trimer with all RBDs down.
A lipid-binding pocket as a selective advantage
In a nutshell, this study confirmed that 'locked' conformation of SARS-CoV-2 (i.e., with all RBDs down) likely represent the prefusion resting state; furthermore, the conformation is compact and stable and braced by lipid-bound within a potentially druggable pocket.
"We have described a well-ordered closed conformation of prefusion spike that appears to result from the incorporation of an endogenous pocket-factor", say study authors. "This conformation is stabilized by a series of specific, conserved interactions which tightly pack the pocket-filled RBD and N-terminal domain around the molecular 3-fold axis", they add.
It appears that RBD interactions induced in this structure stabilize regions of S2 subunit of the spike, preventing the premature conversion of the prefusion state. Crucial neutralization epitopes are shielded in the 'locked' form. At the same time, the loss of lipid may prompt a cascade of events that results in cell entry, akin to the mechanism observed in enteroviruses.
Moreover, the conservation of the residues shaping the pocket the researchers have identified indicates that this locked conformation may occur across the group of beta-coronaviruses as a 'resting conformation,' wielding selective advantage in keeping the immunogenic sites (including the ACE2 binding site) as inaccessible as possible.
"These data, together with the recent observation of a similar structure in wild-type spike ectodomain, strongly suggest that before recognition by a cellular receptor, the 'locked' conformation will be the predominant prefusion form on the viral surface," emphasize study authors.
Implications for drug and vaccine design
But these findings are not only an exercise in satisfying scientific curiosity; instead, the 'locked' spike conformation has considerable potential for therapeutic design. As the release of pocket-bound fatty-acids initiates a conformational change which primes the virion for genome release, any pocket factor mimic that fails to release may be potentially used as a drug.
"In the case of the spike, we suggest that pocket binders which either stabilize or destabilize the spike would offer a plausible route to a therapeutic, and we demonstrate that several compounds significantly destabilize spike," claim study authors in the bioRxiv paper.
Information on the stabilization mechanism of the prefusion spike is also important for understanding the context of epitopes presented in vivo and, consequently, the optimization of presentation in non-classical vaccines.
In summary, these findings are one additional step towards grasping the exact structural dynamics of this complex and fairly unstable molecule – with substantial implications for vaccines and antiviral therapy.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Carrique, L. et al. (2020). The SARS-CoV-2 Spike harbours a lipid binding pocket which modulates stability of the prefusion trimer. bioRxiv. https://doi.org/10.1101/2020.08.13.249177.
- Peer reviewed and published scientific report.
Huo, Jiandong, Yuguang Zhao, Jingshan Ren, Daming Zhou, Helen M. E. Duyvesteyn, Helen M. Ginn, Loic Carrique, et al. 2020. “Neutralization of SARS-CoV-2 by Destruction of the Prefusion Spike.” Cell Host & Microbe, June. https://doi.org/10.1016/j.chom.2020.06.010. https://www.sciencedirect.com/science/article/pii/S1931312820303516.