A research group from Slovenia, Switzerland, and Germany shows how nanoscaffolded assemblies may neutralize interaction between the SARS-CoV-2 spike protein and cell receptor, providing adequate protection in a surrogate infection assay and warranting the advance of such platforms in the pursuit for the effective vaccine. Their results are currently available on bioRxiv* preprint server.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a causative agent of the ongoing COVID-19 pandemic with tremendous casualties and societal cost. At the moment, vaccination seems to be our best hope to stop the waves of infection that are still rampant around the world.
Any effective vaccine should trigger a protective humoral and cell-mediated immune response. Currently, the receptor-binding domain (RBD) found in the surface-exposed spike protein of SARS-CoV-2 is considered a good target for inducing neutralizing antibodies upon vaccination.
The propensity of viral proteins (or its domains) to induce antibody formation depends on the structure and size of the respective antigen. Accordingly, the presentation of antigens (especially their multimeric state) plays a substantial role in generating protective immune response triggered by vaccines.
In recent years, several scaffolds for protein oligomerization with well-defined structures have been utilized for vaccine development against different antigens – including many from viruses (such as influenza and HIV). Furthermore, recent reports on vaccines using RBD showed substantial neutralization and protection properties against the infection.
In this new groundbreaking study, researchers from the Slovenian National Institute of Chemistry, University of Ljubljana, and EN-FIST Centre of Excellence in Slovenia, as well as from the École Polytechnique Fédérale de Lausanne (Switzerland) and University of Regensburg (Germany) compared several strategies for presenting SARS-CoV-2 spike protein RBD on nanoscale particles.
Four scaffolding domains and a neutralization test
In short, the researchers have developed and analyzed the immune response to several DNA vaccines, based on genetic fusions of RBD to four different scaffolding domains. More specifically, the domains were the foldon peptide, lumazine synthase, ferritin, and beta-annulus peptide, harboring between 6 and 60 copies of the RBD on each particle.
SARS-CoV-2 spike pseudotyped virus has been used in the cell assay to test infection in naïve and immunized mice. More specifically, mice were immunized with various combinations of RBD plasmid DNA, and their spleens were harvested at the end of the experiment.
The neutralization of the spike protein engagement with the cell receptor has been investigated by a neutralization test based on the inhibition of their interaction, showing a significant correlation with the neutralization of viral infection.
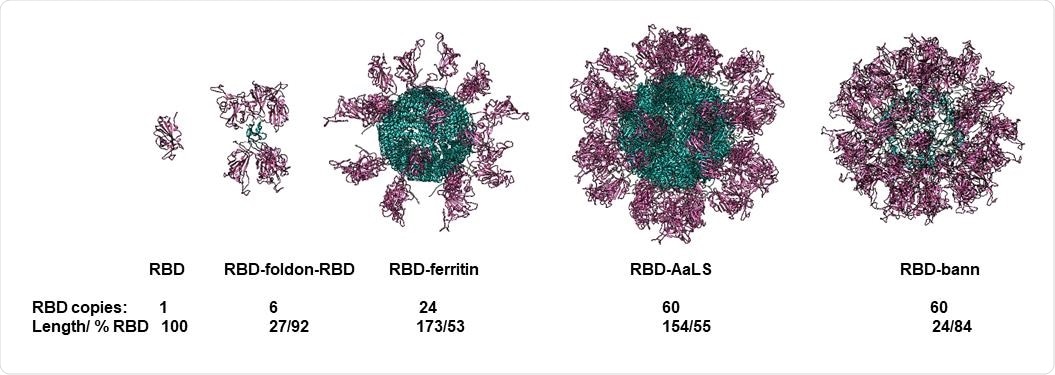
Molecular models of designed scaffolded RBD domains. RBD domains are shown in violet and scaffold core in blue. Number of RBD domains per particle, length of the scaffolding domain and fraction of amino acid residues the RBD in the assembly are written below each model.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
A vigorous immune response
"We show that RBD fused to the scaffolding domains strongly increased the titer of RBD-specific antibodies that also recognized the spike protein, neutralized interaction between the spike and ACE2 receptor, and provided protection in a surrogate infection assay", study authors summarize their findings.
More specifically, scaffolding strongly increased the immune response, as evidenced by the production of neutralizing antibodies and ample T cell response (including cytotoxic lymphocytes) in mice following the immunization procedure with DNA plasmids.
The proportion of the scaffold amino acid residues in the constructs used ranged significantly – from as small as 8% to approximately 50%. Those with the smallest fraction generated a very low level of scaffold-directed antibodies, even when booster shots were given to the test animals.
Interestingly, the response was optimal with the fusion to a beta-annulus peptide that has the ability to produce large soluble oligomers, rather than for other types of large and structurally well-defined scaffolds.
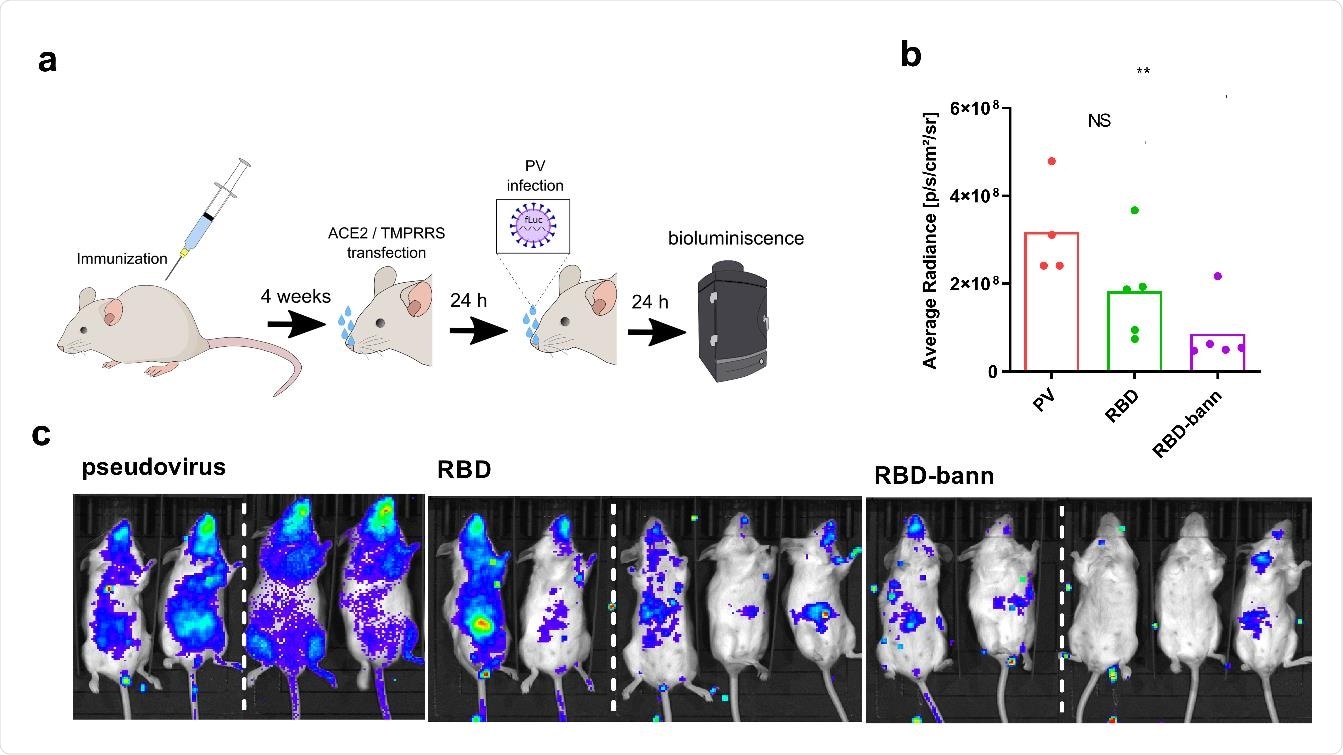
Protection of pseudoviral infection by DNA plasmid immunization in a mouse model. Mice were immunized by two injections of plasmids separated by two weeks. After one month hACE2 and TMPRRS was introduced by intranasal plasmid transfection followed by intranasal infection with SARS_CoV-2 S-typed virus (PV). Luminescence based on pseudovirus intranasal infection was measured after 24 hrs (A). Bioluminescence imaging revealing the protective state of immunized animals against pseudovirus infection in animals. Subsequent quantification of bioluminescence average radiance was carried out (B, C). Dashed line represent merging of pictures of mice from the same test group taken separately. Each dot represents an individual animal (pcDNA3 n=4; RBD and RBD-bann n=5). **P< 0.01. All P values are from Mann-Whitney test.
Clinical trials as a next step
In any case, these results justify the further progress of these types of vaccines into clinical trials. Albeit the researchers have used a DNA delivery platform for inducing the production of oligomeric RBD in the tissue, the same scaffolding strategy may be used for other delivery platforms – including isolated proteins, messenger RNA, and other diverse viral platforms.
"Genetic fusion of target antigen to small scaffolding domain enables fast design and implementation as nucleic acid-based vaccines, particularly in case of epidemic emergencies," conclude study authors in this bioRxiv paper.
To conclude, assemblies that present multiple copies of the RBD (as presented in this paper) are very promising candidates for clinical trials in the continuing pursuit of the vaccine against COVID-19. Nonetheless, they also represent a worthy platform for vaccine development against other potentially emerging viral infections.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Lainšček, D. et al. (2020). Immune response to vaccine candidates based on different types of nanoscaffolded RBD domain of the SARS-CoV-2 spike protein. bioRxiv. https://doi.org/10.1101/2020.08.28.244269.
- Peer reviewed and published scientific report.
Lainšček, Duško, Tina Fink, Vida Forstnerič, Iva Hafner-Bratkovič, Sara Orehek, Žiga Strmšek, Mateja Manček-Keber, et al. 2021. “A Nanoscaffolded Spike-RBD Vaccine Provides Protection against SARS-CoV-2 with Minimal Anti-Scaffold Response.” Vaccines 9 (5): 431. https://doi.org/10.3390/vaccines9050431. https://www.mdpi.com/2076-393X/9/5/431.