The global pandemic of COVID-19 continues to cause thousands of deaths and many hundreds of thousands of new infections every day. New methods are urgently needed to identify severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections and manage them better, including developing criteria for hospitalization.
A new study by a large team of Spanish researchers and published on the preprint server medRxiv* in August 2020 reports that the presence of viral RNA in the blood is a useful marker of impending acute disease and can help triage COVID-19 disease patients who will require hospital care.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Severe COVID-19 Statistics
Among patients in hospital, up to a fifth will require intensive care unit (ICU) admission, up to a tenth need intubation, and up to 5 percent will die of the disease. Most cases are detected by testing for viral RNA in nasopharyngeal swabs, even though it can also be found in sputum, lung fluid, blood, and stool specimens. The lungs are most commonly affected, among the host tissues. However, severe disease can ravage the liver, spleen, lymph nodes, brain, and kidneys.
Viremia Prevalence by COVID-19 Severity
The current study focused on identifying the role of systemic spread of the virus or its components in different organs and in the poorly controlled cytokine release that is often seen in severe COVID-19. The researchers first looked at the link between the presence of the virus in peripheral blood and the disease severity. They then looked at how viremia affected several biological measures of tissue damage and immune hyperactivation.
The study included 250 adults who had all had a positive nasopharyngeal swab test by polymerase chain reaction (PCR) testing during the first wave in Spain, from March 16, 2020, to April 15, 2020. They classified them into three groups: 50 outpatients (hospitalized less than 24 hours), 100 patients hospitalized in the wards but did not develop critical disease, and 100 ICU patients. They examined plasma taken from these patients on collection days 7, 8, and 10, respectively, along with control samples from 20 blood donors, comprised of equal numbers of both sexes.
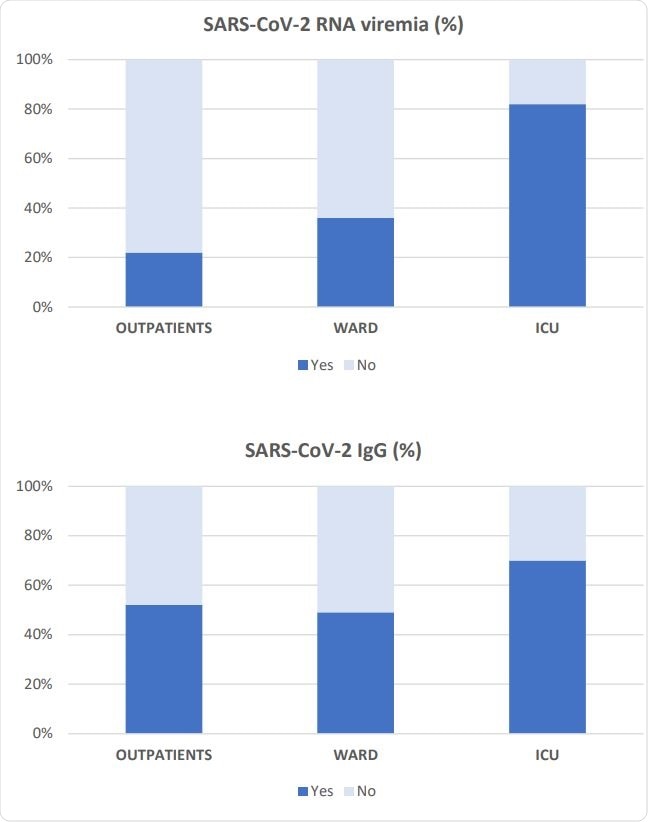
Prevalence of SARS-CoV-2-RNA viremia and SARS-CoV-2 IgG antibodies in each severity group.
Older, Sicker Patients More Prone to Severe COVID-19
They found that most patients who were hospitalized with COVID-19 were older, at a median of 64 and 66 years of age for those in the ward and ICU, respectively, compared to 48 years for outpatients. ICU patients had a larger proportion of male patients. Hospitalized patients had obesity, high blood pressure, abnormal lipid levels, and type II diabetes more often, but these did not differ significantly between groups of hospitalized patients.
Oxygen saturation was markedly lower in ICU patients at admission, all of whom had lung inflammatory infiltrates, and 93% also had pneumonia in both lungs. These figures are significantly higher than in the other groups. Other blood values that were higher in the critical COVID-19 patients include glucose, INR, D-dimers, LDH, ferritin, and C-reactive protein, while the hematocrit value was lower in this group.
Almost half of the ICU patients succumbed to the illness, and their hospital stay was longer. They also received more experimental drugs during their treatment period.
Viremia and Specific IgG Prevalence
The researchers evaluated the frequency of viremia in all three groups. They found that critically ill patients had a higher prevalence of viremia, at over 80%, compared to only 36% and 22% in ward patients and outpatients, respectively. In fact, there was no statistical difference between the latter two groups. The specific IgG antibodies were also found to occur more often in the ICU patients, at 70%, while just over half the patients in the other two groups had specific IgGs against this virus.
Viremia and Disease Severity
The researchers also found that the presence of virus RNA in blood predicted greater severity of illness in any category. Viremia in the ICU group was linked to an eight-fold increase in the odds of severe disease. Among ICU patients, there was no significant difference in the incidence of viremia between those who survived and those who did not.
Viremia and Laboratory Tests
Using multivariate analysis, the researchers found viremia to be an independent marker for elevated ferritin, LDH, and chemokines like CXCL10 and CCL-2, as well as cytokines like IL-15, IL-10, and IL-1ra. It was also associated with lymphopenia, monocytopenia, and thrombocytopenia, along with lower IL-4 levels in plasma. The viremia showed a dose-dependent relationship with the levels of these cytokines and growth factors, with markedly higher levels in critically ill patients compared to ward patients and still more significant differences compared to outpatients.
Is Viral RNA Indicative of Live Virus in Blood?
The finding of viral RNA in the blood may not be synonymous with the presence of the live virus, which is apparently not typically cultured from blood samples. The viral RNA might also be the result of a significant spill-over occurring from infected cells elsewhere. The association of viremia with markers of tissue injury like LDH and low oxygen saturation, which indicates lung damage and respiratory failure, seems to confirm that direct infection by the virus is the cause of the damage.
Viremia Stimulates Cytokine Release?
Secondly, viremia could also contribute to the increased cytokine levels in severe COVID-19, as indicated by the accompanying high chemokine concentrations. Cytokine secretion is enhanced via intracellular signaling pathways, which are activated by the recognition of the RNA by Toll-free receptors like TLR7, found on dendritic cells and B cells, or TLR8 in myeloid cells. A recent paper shows that more RNA fragments of SARS-CoV-2 can be recognized by these receptors than there were for the earlier SARS-CoV, which indicates a correspondingly higher risk of cytokine dysregulation and hyperactive innate immunity.
The current researchers showed in a previous paper that patients with severe SARS had high CXCL10 and CCL2 levels in serum as symptoms set in. They point out that the former of these is a potent chemoattractant molecule and results in Th1 and NK cell activation. This chemokine, therefore, possibly is involved in both innate and adaptive immune responses.
The CXCR3 receptor, on the other hand, is the cognate of the CXCL10 receptor, and it triggers damaging immune responses during other severe viral illnesses like H5N1. The latter was most precisely associated with viremia in the current study and could be developed as a marker of the latter.
CCL2 is a chemokine that controls monocyte migration and infiltration into inflamed tissue. Elevated levels of both CXCL10 and CCL2 in SARS marked the occurrence of lymphopenia in SARS, the latter being found in severe COVID-19 as well.
Vicious Feedback Loop
With severe SARS, there appeared to be a vicious cycle of proinflammatory cytokines that arose as an attempt to mount a proper adaptive immune response to the virus. This could be linked to the levels of IL-10, one of the important immunomodulatory cytokines. Both this and IL1-ra are found to suppress immune responses and are elevated in severe COVID-19, as in the present study, where both were higher in hospitalized patients. The IL-10 levels were higher in ICU patients. These observations may mirror viral immune evasion or a compensatory surge of immunomodulatory chemokines to reduce the excessive magnitude of the immune response.
Other Changes
The rise in IL-15 in viremic patients is a crucial marker of critical disease vs. ward patients. It has a history of being a similarly informative molecule in the flu pandemic of 2009. This pleiotropic cytokine causes T cell proliferation and increased NK cell effector activity, thus being a possible key element of tissue damage caused by T and NK cells. High levels of serum IL-15 are typical in fatal cases.
NK cells in the blood are also high in severe disease, which may be due to IL-15 elevation. In addition, this molecule could also increase the presence of neutrophil extracellular traps (NETs), which may cause endothelial damage and trigger clot formation. Endothelial cells are capable of IL-15 secretion to enhance the migration of both T and NK cells through the endothelial barrier.
High ferritin levels are also common in COVID-19 as a marker of macrophage activation syndrome (MAS), along with elevated cytokines and LDH levels. Blood platelets are also low in MAS, and viremic patients have lower counts than others. In the ICU patients, cases that ended in death had lower platelet counts than survivors at ~180,000 vs 220,000 cell/mm3.
Lymphopenia and monocytopenia may be due to direct viral infection of these cells or due to their recruitment by inflammatory chemokines, or both, and are common to MAS as well as to severe COVID-19.
Implications
The eight-fold increase in the risk of severe disease that accompanies viremia in COVID-19 is independent of age, sex, and the presence of other chronic illnesses.
As a result, the researchers suggest, “Detection of viral RNA in plasma may serve as a simple test to identify those patients needing critical care.’
These findings may indicate that once viral RNA or the live virus enters the bloodstream to reach other tissues, a MAS-like syndrome sets in, which causes severe illness. The virus appears to continue to replicate even as a specific IgG response sets in, as is seen in over 70% of cases. More work is required on linking cellular immune responses with viral replication and disease severity.
However, the researchers point out that “the high prevalence of SARS-CoV-2-RNA viremia in critically ill patients suggests that these patients are unable to control SARS-Cov-2 replication in tissues or blood cells.” This then is a valuable signature, along with the cytokine storm and MAS, of progressive disease, and may indeed be responsible for increasing disease severity.
In such a scenario, the detection of viremia may help to quickly identify patients who have critical disease with just one test. The use of antivirals or convalescent plasma in these patients could cut short the risk, as well as preventing immunological derangement / dysregulation.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources