Although the transmission of SARS-CoV-2, a causative agent of the devastating coronavirus disease (COVID-19) pandemic, primarily occurs via infectious droplets or due to close contact with infected individuals, the virus in the droplet can survive and sustain infectiousness on inanimate surfaces.
Naturally, this contributes to its further spread, and SARS-CoV-2 stability on surfaces under different climate conditions may even be used to predict seasonality of the disease. However, the research in this area has thus far been scarce.
What we do know is that virus stability reduces in human nasal mucus and sputum when compared to culture medium. In contrast, the exposure to simulated sunlight accelerates the viral inactivation on stainless steel. On the other hand, the role of fomites (i.e., inanimate objects that may serve as passive vectors) in the transmission is still heavily debated.
This is why Dr. Taeyong Kwon, Dr. Natasha N. Gaudreault, and Dr. Juergen A. Richt from the Kansas State University in the United States went a step further in their aim to evaluate the behavior and exact biological half-life of SARS-CoV-2 in an indoor setting, summer and spring/fall conditions.
Air-dried virus, different surfaces, and cell lines
This group of researchers tested SARS-CoV-2 stability on twelve different material surfaces – including nitrile gloves, N95 mask, cloth, Tyvek Styrofoam, concrete, cardboard, glass, rubber, polypropylene, galvanized steel, and stainless steel.
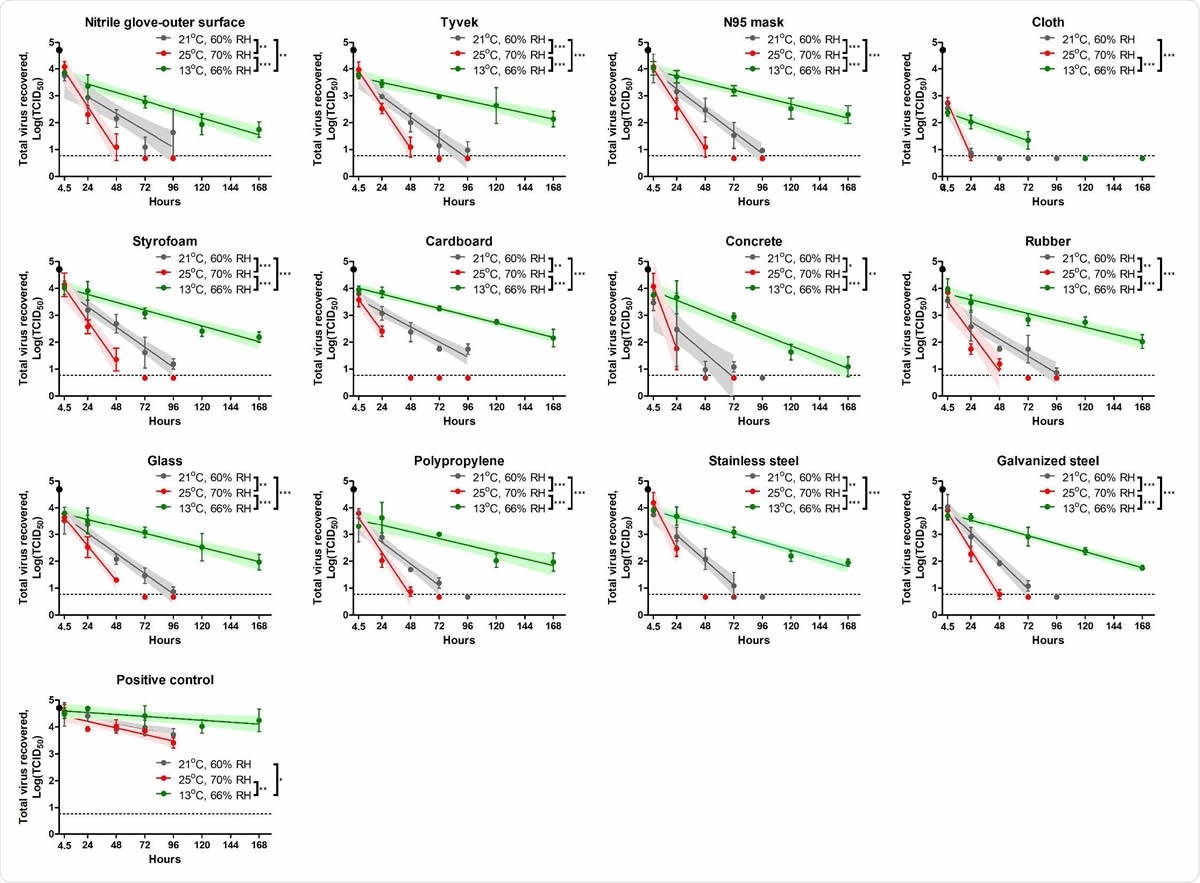
Stability of severe acute respiratory coronavirus 2 (SARS-CoV-2) on different types of surfaces. Each figure represents the virus decay on each surface. Total 50 μl of virus inoculum (5×104 TCID50, black dot) was added onto each material and dried for 4.5 hours inside a biosafety cabinet. The virus survival was evaluated under three different conditions: at 21°C/60% RH (grey), 25°C/70% RH (red) and 13°C/66% RH (green). The infectious virus was recovered at 4.5 (after drying period), 24, 48, 72, and 96 hours post-contamination (hpc) at 21°C/60% RH and 25°C/70% RH and 4.5, 24, 72, 120, and 168 hpc at 13°C/66% RH. Virus titer at each time point was expressed as mean log10 transformed titer with standard deviation. Linear regression models were estimated; the solid line and its shade area represent an estimated best fit model and 95% confidence intervals, respectively. Limit of detection (LOD) in each titration assay was 100.968 TCID50 and a negative result is represented as a half value of LOD, 100.667 TCID50. The dash line shows LOD in triplicate, 100.767 TCID50, when there was LOD in one replicate, but negative in two other replicates. Statistical significance between two slopes of linear regression models is represented as * (p < 0.05), ** (p < 0.01), *** (p < 0.001).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The virus used in the experiments was air-dried inside a biosafety cabinet for approximately 4.5 hours. Then the plate containing virus-contaminated material has been incubated under three different environmental conditions simulating indoor setting, summer, and spring/fall conditions characteristic for the Midwestern United States.
Finally, the recovered virus was titrated on Vero (African green monkey kidney) cells, while viral titers were calculated by using the Reed-Muench method, which is a simple tool in experimental biology for determining 50% endpoints or the test substance concentration that produces an effect in half of the test units.
Differing half-life ranges
This study showed that the longest half-life of SARS-CoV-2 can be observed during spring and fall conditions (13 °C with relative humidity of 66%), ranging from 17.11 to 31.82 hours.
This is followed by indoor conditions (21°C with relative humidity of 60%) and summer conditions (25°C with relative humidity of 70%), where viral half-lives were 3.5-11.33 hours and 2.54-5.58 hours, respectively.
"We found the half-life on most surfaces at 21°C/60% relative humidity is 6.93–12.86, but the virus is quickly inactivated on cloth with a 3.5 hours half-life", further state study authors in this bioRxiv paper.
Such noticeable difference might be explained by the organization of the virus inoculum used in the study, the volume of inoculum, different environmental conditions, as well as differences in the material preparation.
Public health implications
"Our study showed a remarkable persistence of infectious SARS-CoV-2 on various types of surfaces, especially under spring/fall climate conditions", say study researchers. "However, virus stability was highly dependent on the substrate as well as temperature and humidity", they add.
Prolonged survival on various surfaces in spring, fall and winter might support viral transmission through contaminated fomites and possibly contribute to new outbreaks or seasonal occurrence in the post-pandemic era – a scenario already seen for influenza virus and other human coronaviruses.
Consequently, this reinforces the importance of adequate personal hygiene and consistent disinfection of potentially contaminated surfaces, which remains a pivotal tool to minimize the risk of infection via contaminated surfaces.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources