COVID-19, the acute illness due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in Wuhan, Hubei province, China, in December 2019, and rapidly progressed to a global pandemic. As of today, a total of 25.48 million people have been infected with this virus, and over 850,000 have died. Although non-pharmaceutical interventions such as quarantine, isolation, and social distancing have, to some extent, countered the spread of SARS-CoV-2, countries now face a multitude of challenges to the “re-opening” of society. It is evident the only way to provide effective herd immunity is with a safe and effective vaccine. Now, a new study published on the preprint server bioRxiv* in August 2020 reports the induction of neutralizing antibodies in mice by a single dose of a ferritin nanoparticle-based vaccine containing the spike protein.
SARS-CoV-2 enters the host cell by binding via its spike protein to the angiotensin-converting enzyme 2 (ACE), the host cell receptor. The spike protein exists as a trimer, with two subunits, S1 and S2, that play a role in viral attachment and fusion, respectively. The S1 subunit has a receptor-binding domain (RBD) that undergoes functional folding when separated from the rest of the subunit.
Recovered COVID-19 patients show high titers of neutralizing antibodies directed against this protein, indicating that it could be used to produce a protective vaccine. Indeed, the spike glycoprotein has been the focus of intensive vaccine development efforts.
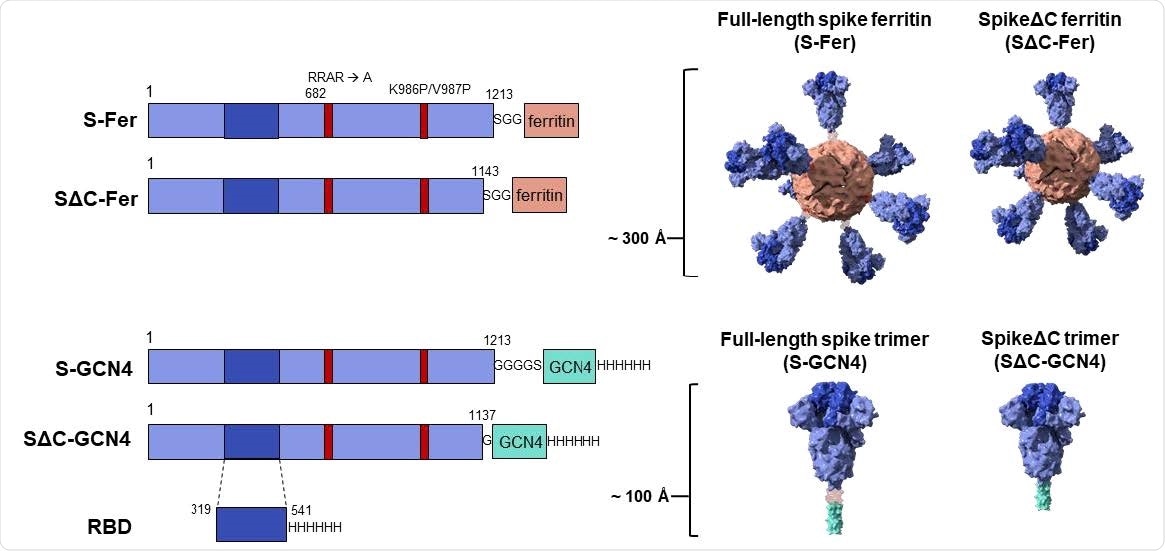
Construct design for SARS-CoV-2 spike-functionalized ferritin nanoparticles. All constructs are based on the Wuhan-Hu-1 amino acid sequence (GenBank MN9089473) of SARS-CoV-2 spike. Spike-functionalized ferritin constructs were made by fusing spike ectodomain (residues 1-1213) or spikeΔC (residues 1-1143) to the H. pylori ferritin subunit separated by an SGG linker. A structural representation based on the spike trimer cryo-EM structure (PDB 6VXX) and the H. pylori ferritin crystal structure (PDB 3BVE) depicts the 24-subunit particle displaying spike or spikeΔC on the surface. The estimated size of the spike-functionalized ferritin particles based on structural data is ~ 300 Å. The S-GCN4 and SΔC-GCN4 trimer constructs were made by fusing either the full-length spike residues (1-1213) or spikeΔC (1-1137) to a modified GCN4 trimerization domain followed by a hexahistidine tag. A structural representation of the spike trimers based on the cryo-EM structure (PDB 6VXX) is shown with an estimate length of ~ 100 Å. The RBD spans residues 319-541 of the spike protein and is preceded by the native signal peptide (not shown) and followed by a hexahistidine tag.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Advantages of a Subunit Vaccine
The current study focuses on the production of a subunit vaccine, rather than a virus-based vaccine. The reasons for this choice include improved storage stability, which includes a greater range of distribution, ease of manufacture, safety, and greater consistency in the vaccine quality. However, the limiting factor is the weaker immune response associated with them.
Ferritin-Based Vaccine
To overcome this, adjuvants are typically added to enhance their immunogenicity. However, presenting the antigen in a multivalent format is also an effective way to achieve this, and the use of nanoparticles is one such approach. The current study uses Helicobacter pylori ferritin, which has been employed to display antigens from the influenza virus, HIV, and Epstein-Barr virus.
The H. pylori ferritin is a protein that displays the property of self-assembly, forming particles containing 24 subunits, with eight 3-fold symmetries. This enables it to fuse with one spike protomer per subunit, allowing the display of eight trimeric spike antigen subunits at the surface of the assembled particle. Moreover, earlier research shows that antigens mounted on ferritin induce greater immunity compared to immunization with the antigen alone.
Two vaccines using influenza virus proteins on a ferritin platform have been demonstrated to be clinically viable in clinical trials. The facilities for large-scale ferritin vaccine manufacture are also ready.
Different Spike-Based Vaccine Designs
In the current study, the researchers used two designs to construct ferritin vaccines. One, they used the full-length spike ectodomain, while in the second case, they used it after deleting the 70 residues at the C-terminal end of the ectodomain. The reason for this deletion was the possible conformational flexibility in this region and the presence of a linear epitope, which elicited a robust immune response.
These were attached to ferritin to form S-Fer and SΔC-Fer, respectively. Both were stabilized in the prefusion conformation, which leads to better expression and enhanced immunogenicity. As controls, they also used three other antigens, namely, a spike trimer with a trimerization domain in full-length or SΔC form, and a monomeric RBD.
Functionalized Ferritin Shows No Disadvantages
They found that spike-ferritin nanoparticles can be produced within mammalian cells in culture and purified to obtain a homogeneous result. The fusion of the ferritin to the spike protein did not adversely impact protein expression; indeed, SΔC-Fer actually showed improved expression. The functionalization of the H. pylori ferritin with the spike did not disrupt the nanoparticle self-assembly, either.
After checking the ferritin constructs for stability and homogeneity, they examined the folding using various tests for biophysical, structural, and binding properties. They employed size-exclusion chromatography multi-angle light scattering (SEC-MALS), cryo-electron microscopy (cryo-EM), and bio-layer interferometry (BLI) to evaluate these characteristics. They found that the functionalized nanoparticles displayed stable folding, and the epitopes of interest were demonstrated properly. They also found that the spike-functionalized particles were bound by both the virus and ACE2 to the same extent as trimeric spike proteins and the RBD.
Effective Immune Response After Single Dose
The researchers then immunized mice with a single dose of these vaccines to study the immune response in vivo. Using pseudotyped viruses to avoid biosafety issues, they confirmed that SΔC-Fer elicited a markedly higher neutralizing antibody response compared to all the non-ferritin vaccines. Both S-Fer and SΔC-Fer vaccines induced neutralizing antibodies at double the titer following natural infection.
The researchers comment, “These results demonstrate that spike-functionalized ferritin nanoparticles elicit an enhanced antibody response compared to the spike trimers or RBD alone.”
Second Dose Improves Efficacy for Other Vaccine Candidates
A second dose of the five antigens on day 21 showed that the immune response was boosted for all groups to an almost complete blocking of ACE2 at a serum dilution of 1:50. Another observation was that ACE2 blocking is not always correlated to the trend of neutralizing activity. This could be because other neutralizing epitopes are present on the spike glycoprotein. However, SΔC-Fer-immunized mice continued to show the highest neutralizing titers, showing that a multivalent spike presentation is necessary for a better and more reliable immune response.
Implications
The conclusion is that these spike ferritin nanoparticles are superior candidates for vaccine development to either spike trimer or RBD by themselves, and that, in their words, “SΔC-Fer is the best-performing antigen out of those we tested here.”
The high efficacy of a vaccine candidate in achieving neutralizing antibody response after a single dose could be the tipping point in achieving worldwide coverage and stopping the pandemic. Thus, this study gains more importance in demonstrating the favorable profile of this construct in developing an effective and widely deployable vaccine against COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Powell, A. E. et al. (2020). A Single Immunization with Spike-Functionalized Ferritin Vaccines Elicits Neutralizing Antibody Responses Against SARS-Cov-2 In Mice. bioRxiv preprint. doi: https://doi.org/10.1101/2020.08.28.272518. https://www.biorxiv.org/content/10.1101/2020.08.28.272518v1
- Peer reviewed and published scientific report.
Powell, Abigail E., Kaiming Zhang, Mrinmoy Sanyal, Shaogeng Tang, Payton A. Weidenbacher, Shanshan Li, Tho D. Pham, John E. Pak, Wah Chiu, and Peter S. Kim. 2021. “A Single Immunization with Spike-Functionalized Ferritin Vaccines Elicits Neutralizing Antibody Responses against SARS-CoV-2 in Mice.” ACS Central Science 7 (1): 183–99. https://doi.org/10.1021/acscentsci.0c01405. https://pubs.acs.org/doi/10.1021/acscentsci.0c01405.