Researchers in the United States have demonstrated that a drug originally developed as a treatment for cancer exhibits antiviral activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the agent that causes coronavirus disease 2019 (COVID-19).
The drug, which is called masitinib, potently inhibited the main viral protease 3CLpro that SARs-CoV-2 relies on for infection to proceed once the viral genome has entered the host cell cytoplasm.
The study showed that masitinib directly bound to the active site of 3CLpro and blocked its ability to cleave the viral polyprotein into the individual proteins that are needed for infection to be successful.
Savaş Tay from the University of Chicago and colleagues say that, as well as being a strong candidate for the treatment of SARS-CoV-2 infection, masitinib also showed antiviral activity against the related picornaviruses. These agents cause a wide range of diseases in humans and other mammals, including meningitis, hepatitis, and poliomyelitis.
A pre-print version of the paper is available in the server bioRxiv*, while the article undergoes peer review.
The Coronaviridae family
Since the first cases of COVID-19 first occurred in Wuhan, China, late last year, SARS-CoV-2 has swept the globe and now infected more than 25.86 million people and caused more than 859,000 deaths.
SARS-CoV-2 is a betacoronavirus that belongs to the Coronaviridae family. This family also includes the betacoronaviruses SARS-CoV-1 and Middle East respiratory syndrome coronavirus (MERS-CoV) and four other endemic pathogens that are responsible for the common cold.
The drug development process generally takes several years
Once the viral genome of SARS-CoV-2 has reached the host cell cytoplasm, it needs to be translated into a polyprotein that can be cleaved into the viral proteins required for infection to proceed. The main viral enzyme that is responsible for this cleavage is 3CLpro and there is much interest in developing drug inhibitors of this protease.
However, the drug development process is time-consuming and usually takes several years, say Tay and colleagues.
Furthermore, many promising vaccine candidates have entered clinical trials. Still, their success cannot be guaranteed and, even if a vaccine was approved, producing and administering it at the levels needed to control the current pandemic will also take a long time.
“Therefore, there is a need for new treatment options for COVID-19,” say Tay and colleagues.
“One obvious to speed up drug discovery”
Although the antiviral remdesivir did receive FDA emergency use authorization, once it was shown to decrease disease severity and shorten hospitalization times among COVID-19 patients, there are currently no FDA-approved specific antivirals for the treatment of SARS-CoV-2 infection.
“The introduction of additional antivirals would help to reduce morbidity and mortality,” say the researchers. “One obvious way to speed up drug discovery is through the use of drug-repurposing screens, looking to identify safe-in-human drugs with potential anti-coronavirus properties.”
What did the researchers do?
First, Tay and team screened a library of 1,900 clinically established drugs for their ability to inhibit the replication of a human betacoronavirus called OC43 (HCoV-OC43). This virus, which causes the common cold, is a relative of SARS-CoV-2.
After identifying 26 effective drugs, the researchers determined their ability to inhibit the enzymatic activity of 3CLpro, as well as other pathogenic viruses.
The team identified 20 drugs that significantly inhibited the replication of SARS-CoV-2 in a human lung cell line.
On investigating the underlying mechanism of action, the researchers found that the Tyrosine Kinase Inhibitor (TKI) masitinib, which was initially developed as a cancer treatment, potently inhibited 3CLpro.
X-ray crystallography revealed that masitinib binds directly to the active site of 3CLpro, thereby blocking its enzymatic activity.
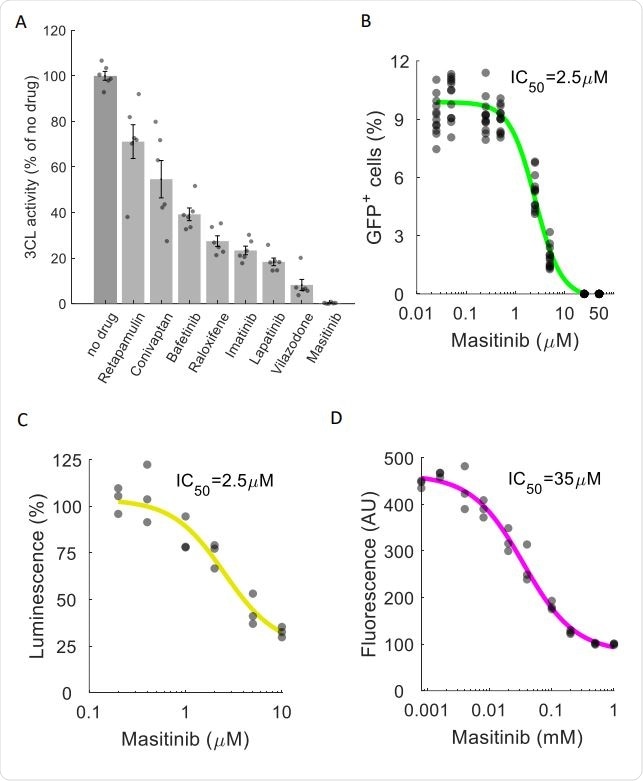
Masitinib inhibits SARS-CoV-2 3CLpro enzymatic activity. A. A FlipGFP reporter assay was performed to screen for potential inhibition of 3CLpro by the identified drugs at a single concentration (10µM). Shown are the drugs that showed a statistically significant reduction in 3CLpro activity (pvalue<0.05, one-tailed t-test, FDR-corrected). n=6. The data for the remaining tested drugs is shown in Figure S4. Individual measurements are shown in circles. Bars depict mean ± s.e. Masitinib treatment completely inhibited 3CLpro activity. B. Dose-response curve for 3CLpro inhibition by masitinib using the FlipGFP reporter assay, n = 6. Individual measurement shown as circles. C. Dose-response curve for 3CLpro inhibition by masitinib using a luciferase reporter assay, n = 3. Induvial measurement shown as circles D. Dose-response curve for 3CLpro inhibition in a cell-free assay using purified 3CLpro and a flurogenic peptidic substrate, n = 3. Individual measurement shown as circles.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Other important beneficial effects of masitinib
The researchers say it is important to note that, as well as the direct-antiviral effect that was observed here, masitinib has previously been shown to reduce inflammation in the airways and improve lung function in an animal model of asthma.
“Given that a main pathology of SARS-CoV-2 is ARDS (acute respiratory distress syndrome), the combined antiviral and anti-inflammatory properties of masitinib might prove highly beneficial for treating COVID-19,” suggests the team.
Furthermore, the study found that masitinib also inhibited a viral protease of picornaviruses - pathogens related to SARS-CoV-2 - and blocked their ability to replicate.
Masitinib, a strong candidate for the treatment of SARS-CoV-2 infection
The researchers say their findings have shown that HCoV-OC43, a pathogen that can be readily studied in most virology labs, serves as a good model for screening potential antiviral drugs against SARS-CoV-2 infection.
“Our results show that masitinib has broad antiviral activity against two distinct beta-coronaviruses and multiple picornaviruses that cause human disease and is a strong candidate for clinical trials to treat SARS-CoV-2 infection,” concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Tay S, et al. Drug repurposing screen identifies masitinib as a 3CLpro inhibitor that blocks replication of SARS-CoV-2 in vitro. bioRxiv, 2020. doi: https://doi.org/10.1101/2020.08.31.274639
- Peer reviewed and published scientific report.
Drayman, Nir, Jennifer K. DeMarco, Krysten A. Jones, Saara-Anne Azizi, Heather M. Froggatt, Kemin Tan, Natalia Ivanovna Maltseva, et al. 2021. “Masitinib Is a Broad Coronavirus 3CL Inhibitor That Blocks Replication of SARS-CoV-2.” Science, July, eabg5827. https://doi.org/10.1126/science.abg5827. https://www.science.org/doi/10.1126/science.abg5827.