MIS-C is a condition that sometimes develops among individuals aged 6 to 21 years following infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the agent that causes coronavirus disease 2019 (COVID-19).
The study revealed an “MIS-C signature,” where genes that were downregulated in cases of MIS-C were clustered in a module that would code for exhausted CD8+ T Cells and a subset of natural killer (NK) cells.
The study also revealed key gene regulators within this module, including a gene called TBX21, which is an essential coordinator of CD8+ T-cell differentiation.
Overall, the findings point to a relationship between a dysregulated cytotoxic lymphocyte response to SARS-CoV-2 infection and MIS-C, say Alexander Charney and team.
A pre-print version of the paper is available in the server medRxiv*, while the article undergoes peer review.
MIS-C is distinct from COVID-19

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
MIS-C develops in people aged under 21 years and is characterized by fever, inflammation, and dysregulation of at least two organ systems, including cardiac, respiratory, renal, neurological, and gastrointestinal. Although the condition develops following SARS-CoV-2 infection, it is clinically distinct from COVID-19.
MIS-C shares symptoms with another pediatric inflammatory condition called Kawasaki disease (KD), which is characterized by acute vasculitis and inflammation.
Due to the similarity in symptoms between these two conditions, MIS-C was initially termed “Kawasaki-like” disease. However, the similarity between MIS-C and Kawasaki’s disease at the molecular level has not yet been established.
Furthermore, researchers had proposed that MIS-C is a systemic autoimmune disease that is triggered by SARS-CoV-2 infection, but the underlying pathogenesis of the condition has yet to be appropriately determined.
What did the researchers do?
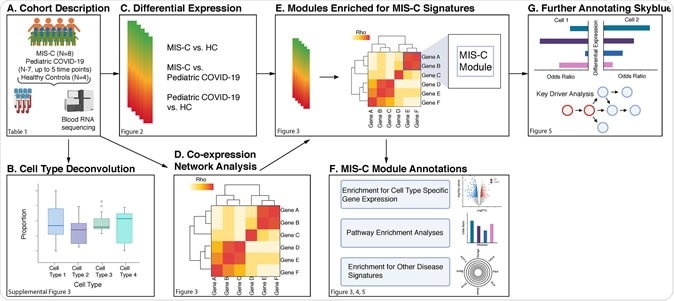
Now, Alexander Charney and colleagues have performed RNA sequencing of whole blood samples from 8 individuals with MIS-C, 7 pediatric COVID-19 cases, and 4 healthy controls to identify the genes, pathways, and cell types that drive MIS-C.
The team built a gene-co-expression network from 30 blood samples taken from the children with MIS-C, to further test the molecular architecture of the MIS-C signature for relationships between MIS-C pathogenesis and Kawasaki disease, SARS-CoV-2 infection, and classic autoimmune diseases.
By projecting the MIS-C signature onto the co-expression network, the team identified 11 gene modules (genes arranged into coherent units) that were enriched for differentially expressed genes. The modules were also enriched for Kawasaki disease and COVID-19 signatures, but there was no overlap with the transcriptional signatures of classic autoimmune diseases.
One module called “skyblue” exhibited the most robust enrichment for downregulated MIS-C differentially expressed genes and showed specific enrichment for transcriptional signatures of exhausted CD8+ T-cells and a subset of natural killer cells called CD56dimCD57+ NK cells.
Finally, probabilistic causal network modeling revealed nine key regulators of this skyblue module, including TBX21, an important coordinator of exhausted CD8+ T-cell differentiation.
“This stepwise approach shed light on several questions regarding the underpinnings of MIS-C,” writes the team. “It revealed a partially shared molecular etiology with KD [Kawasaki disease], but not other pediatric inflammatory conditions, no overlap with the transcriptional signatures of classic autoimmune diseases, and a direct link with the immune response to SARS-CoV-2 infection.”
MIS-C and Kawasaki disease are not necessarily the same
The researchers say the findings support the notion that although MIS-C and Kawasaki disease share similarities, they are not necessarily the same disease.
One module called “paleturquoise,” for example showed a strong enrichment for upregulated genes in MIS-C, platelet markers, and coagulation pathways but a lack of enrichment for the Kawasaki disease signature. This suggests that Kawasaki Disease coagulopathies and MIS-C coagulopathies occur via different molecular mechanisms.
“Overall, the molecular comparison between MIS-C and KD emphasized similarities and differences that may account for their being clinically alike yet distinct,” say the authors.
Checking for the similarity between MIS-C and autoimmune conditions
The team also performed a transcriptome-wide assessment to test the hypothesis that MIS-C shares molecular pathophysiology with classic autoimmune diseases.
Charney and colleagues did not find any co-expression modules that were enriched for both MIS-C and autoimmune disease signatures.
By contrast, they did identify a relationship between SARS-CoV-2 infection and MIS-C at the gene expression level, with several modules enriched for MIS-C and both pediatric and adult COVID-19 differential expression signatures.
While this does not prove causality, it does point to a direct molecular link between MIS-C and SARS-CoV-2, in both the acute and convalescent stages of infection, say the researchers.
Important implications beyond pediatric cohorts
The authors say understanding the relationship between SARS-CoV-2 and MIS-C may have important implications beyond pediatric cohorts. Some convalescent adults report the lingering of symptoms such as fatigue and labored breathing and the team suspects the cytotoxic lymphocyte dysregulation observed here may be a contributing factor.
“As independent cohorts are assembled, replication and validation of our primary findings in blood and other tissues will be paramount,” conclude Charney and colleagues.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Charney A, et al. Cytotoxic lymphocytes are dysregulated in multisystem inflammatory syndrome in children. medRxiv, 2020. doi: https://doi.org/10.1101/2020.08.29.20182899
- Peer reviewed and published scientific report.
Beckmann, Noam D., Phillip H. Comella, Esther Cheng, Lauren Lepow, Aviva G. Beckmann, Scott R. Tyler, Konstantinos Mouskas, et al. 2021. “Downregulation of Exhausted Cytotoxic T Cells in Gene Expression Networks of Multisystem Inflammatory Syndrome in Children.” Nature Communications 12 (1). https://doi.org/10.1038/s41467-021-24981-1. https://www.nature.com/articles/s41467-021-24981-1.