The COVID-19 pandemic has caused hundreds of thousands of deaths, mostly from terminal respiratory failure or multi-organ dysfunction, or other complications. The crisis has called forth intensive scientific efforts to find effective antivirals and vaccines against SARS-CoV-2, since the only option now available to contain the epidemic is non-pharmaceutical interventions relying on physical distancing.
Even as some vaccine candidates enter clinical trials, large-scale production remains a stumbling block in making the vaccine available to the whole world. Multi-dose regimes also pose a challenge in this regard, leading some researchers to focus on the live attenuated vaccine (LAV).
However, another approach is to use a platform that simulates the immunogenicity of the live virus vaccines and can achieve adequate immunity with just one dose. This has the twin advantage of not only reducing logistical obstacles but increasing the odds of compliance and reducing the number of doses required for full compliance.
One such technology is the RNA vaccine, which is based on using the genetic sequence of the viral antigens and can be rapidly produced by cell-free techniques with excellent scalability. The RNA is packaged by lipid nanoparticles, which avoids the need for adjuvants. The two types of RNA vaccine include the conventional mRNA vaccine consisting of a directly translated immunogen derived from the vaccine transcript, and a replicon RNA vaccine that self-replicates.
Introducing Replicon RNA Vaccines
How replicon vaccines works are by means of the encoded machinery for replication, that allow the subgenomic RNA to be self-amplified. This results in a marked increase in the amount of the target antigen, by orders of magnitude. This not only reduces the required vaccine dosage but can induce both innate and adaptive immunity like a LAV.
The current study by researchers at the Duke-NUS Medical School, Singapore and Arcturus Therapeutics, Inc. shows how such a vaccine performs against a conventional mRNA vaccine. The former is built on the proprietary Self-Transcribing and Replicating RNA (STARRTM) technology of Arcturus.
The novel vaccine called LUNAR-COV19, as well as the conventional mRNA vaccine, code for the full-length pre-fusion spike protein in the wildtype virus.
Longer and Higher Immunity
The researchers found that the former induced higher antigen expression in vivo for a significantly more extended period. It also increased the expression of several innate immune genes regulating B and T cell response in the blood and the draining lymph nodes.
Higher Neutralizing Activity
This was associated with significantly higher neutralizing activity as well as the induction of specific IgGs, CD8+ T cells, interferon-(IFN-)γ, and Th1 responses. Antibodies were detected for up to day 50 after immunization, as against day 10 in the case of mRNA.
Neutralizing activity was measured by the plaque reduction neutralization test (PRNT50). It was observed to be greater than the upper limit of dilution with the novel vaccine, which is more than 16 times greater than the neutralization titers achieved with the conventional mRNA vaccine. Moreover, the titers continued to show an upward trend from day 30 to 60 with one dose of vaccine, even with a dose of ≥2.0 μg. The level of spike IgG was positively correlated with the PRNT50 titer as well with the new vaccine candidate compared to the conventional mRNA vaccine.
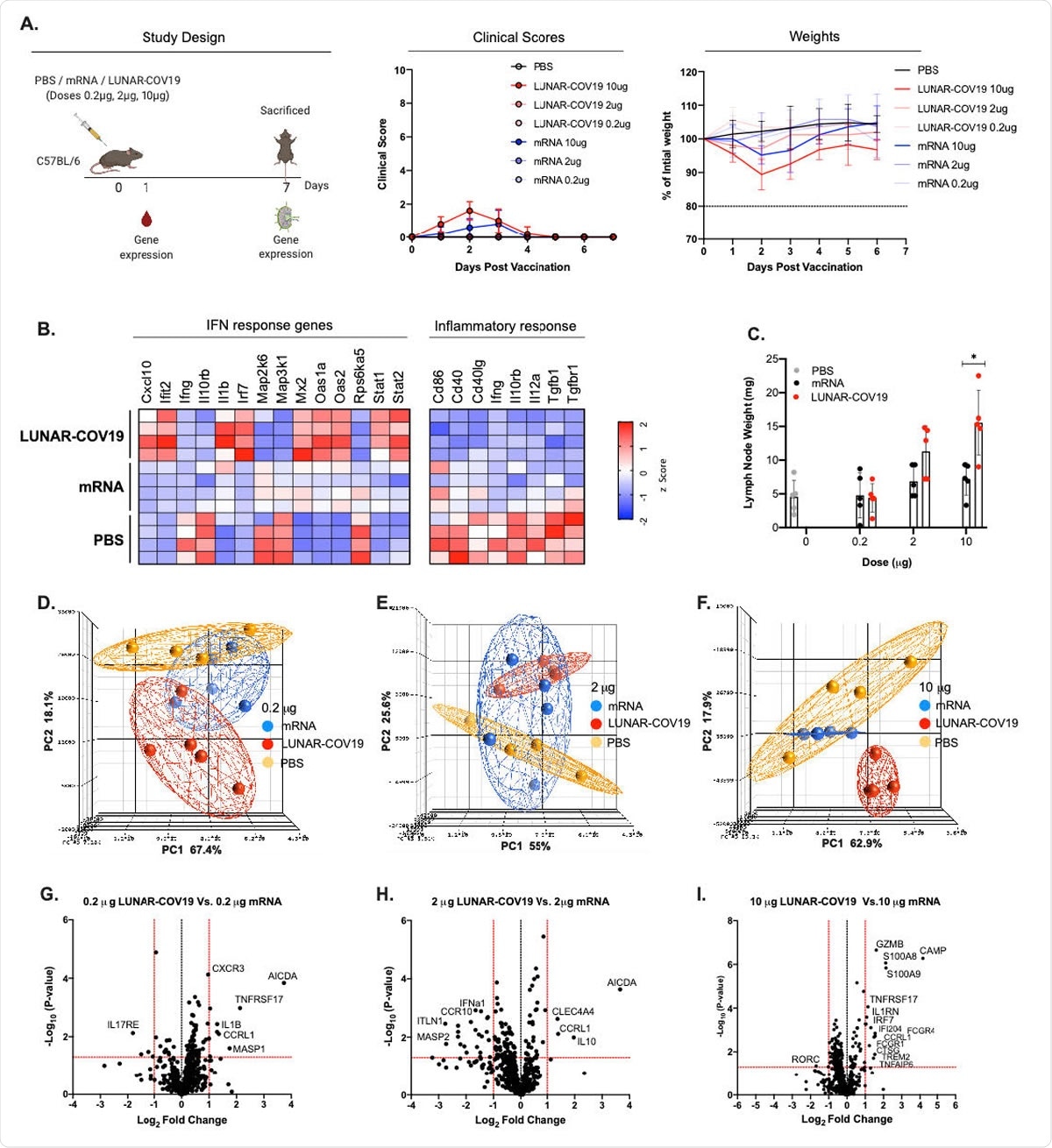
Clinical Scores, mouse weights and transcriptomic analysis of immune genes following vaccination with LUNAR-COV19 or conventional mRNA SARS-CoV-2 vaccine candidates. A) C57BL/6 mice (n=5/group) were immunized with either PBS, mRNA or LUNAR-COV19 (doses 0.2 µg, 2 µg or 10 µg), weight and clinical scores assessed every day, bled at day 1 post-immunization, sacrificed at 7 days post-vaccination and lymph nodes harvested. Gene expression of inflammatory genes and immune genes were measured in whole blood (at day 1) and lymph nodes (at day 7), respectively. B) Expression of IFN and inflammatory response genes in whole blood presented as heatmap of z scores. C) Lymph node weights at 7 days post-vaccination. Principal component analysis (PCA) of immune gene expression following vaccination with conventional mRNA or LUNAR744 COV19 at doses D) 0.2 µg, E) 2 µg and F) 10 µg. Volcano plots of fold change of LUNAR745 COV19 versus conventional mRNA (x-axis) and Log10 P-value of LUNAR-COV19 versus conventional mRNA (y-axis) for doses G) 0.2 µg, H) 2 µg and I) 10 µg. Study design schematic diagram created with BioRender.com. Weights of lymph nodes were compared between groups using a two-tailed Mann-Whitney U test with * denoting 0.05<0.01.
Safer Immune Responses
A Th1-biased immune response is known to be linked to protection against vaccine-associated immune enhancement of respiratory disease (VAERD), which is a paramount safety concern with SARS-CoV-2 vaccines.
Increased Avidity
Again, at the highest dose of conventional mRNA vaccine that was able to produce anti-S antibodies at a level similar to that produced at the lowest level of the novel vaccine, the former elicited antibodies with lower avidity and less neutralizing power than the latter. This indicates that the latter produces a more protective antibody response and with B cells that have a better affinity for the antigen, showing it to be immunologically superior to the conventional mRNA vaccine.
Complete Protection Against Infection
The mice which received a single dose of the vaccine were protected against subsequent infection upon exposure to the virus. However, all the unimmunized controls died of the infection by day 7 of the challenge with wildtype SARS-CoV-2.
Mechanisms of Protection
The reasons for the superior performance of this vaccine may be due to the increased and more prolonged immunogenic expression of the antigen, which is recognized by T follicular helper cells more effectively. This, in turn, causes the targeting of a broader spectrum of viral antigens, presenting a greater range for neutralization and so better neutralizing ability.
Secondly, the replication of this vaccine RNA causes a negative-strand template to form, which leads to the synthesis of a more significant amount of positive-strand mRNA as well as sub-genomic mRNA encoding the spike protein. The two RNA strands form an intermediate double-stranded form that can stimulate the immune pathways mediated by type I IFN through its interaction with receptors like TLR3 and RIG-I-like receptors. These are known to enhance the quality of adaptive immune responses.
Finally, IFN-γ can induce the production of cytotoxic CD8 T cells, which is important since the spike protein does have epitopes that are recognized by these T effector cells. Memory T cells ensure durable immunity.
The replication complex is formed by Venezuelan equine encephalitis virus (VEEV) replicase genes, encoding nsp1-4, which may or may not be immunogenic. However, it is established that nsp mutations do affect type I IFN responses. Also, VEEV self-replicating RNA also enhances mucosal immune responses, similar to adjuvants.
Implications
All these findings show that the STARR platform is worthy of further evaluation in the search for a suitable COVID-19 vaccine, with the novel vaccine reported here having great potential to be developed as a single-dose vaccine.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources