Researchers in the United States and Germany have conducted a study demonstrating the preclinical efficacy of a new candidate vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the agent that causes coronavirus disease 2019 (COVID-19).
Ugur Sahin (University Medical Center of the Johannes Gutenberg University, Mainz, Germany) and colleagues say their preclinical study has now shown that the vaccine is highly immunogenic in mice and rhesus macaques.
In mice, just one injection of the BNT162b2 induced high titers of neutralizing antibodies against SARS-CoV-2 and potent interferon (IFN) and T-cell responses that the authors thought could be protective against infectious challenge.
In rhesus macaques, prime-boost vaccination with BNT162b2 induced neutralizing antibody titers that were up to 18 times higher than those generated when serum from convalescent humans was used.
The new vaccine candidate also protected the lungs of rhesus macaques from infectious challenge with SARS-CoV-2.
A pre-print version of the paper is available on the server bioRxiv*, while the article undergoes peer review.
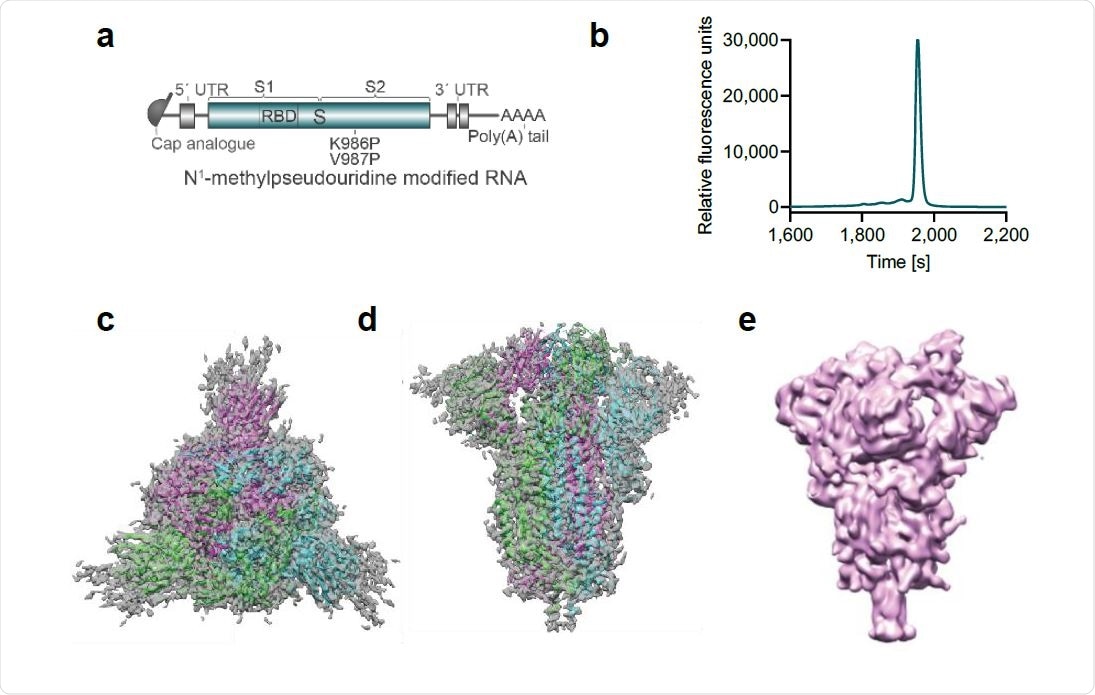
Vaccine design and characterization of the expressed antigen. a, BNT162b2 RNA structure. UTR, untranslated region; S, SARS-CoV-2 S glycoprotein; S1, N-terminal furin cleavage fragment; S2, C-terminal furin cleavage fragment; RBD, receptor-binding domain. Positions of the P2 mutation (K986P and V897P) are indicated. b, Liquid capillary electropherogram of in vitro transcribed BNT162b2 RNA. c, A 3.29 Å cryoEM map of P2 S, with fitted and refined atomic model, viewed down the three-fold axis toward the membrane. d, Cryo-EM map, and model of (d) viewed perpendicular to the three-fold axis. e, Mass density map of TwinStrep-tagged P2 S produced by 3D classification of images extracted from cryo-EM micrographs with no symmetry averaging. This class, in the one-RBD ‘up’, two RBD ‘down’ positioning, represents 20.4% of the population.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The race to develop vaccines against SARS-CoV-2
Since the first cases of coronavirus disease 2019 (COVID-19) were first identified in Wuhan, China, late last year, researchers have been racing to develop safe and effective vaccines that will help to protect against SARS-CoV-2 infection.
In order to infect host cells, SARS-CoV-2 uses a viral surface protein called Spike to bind to the human receptor angiotensin-converting enzyme 2 (ACE2).
The Spike protein binds ACE2 using a receptor-binding domain (RBD) that forms part of its N-terminal furin cleavage fragment (S1). The Spike protein then uses fusion machinery contained in the C-terminal furin cleavage fragment (S2) to fuse to the cell membrane and deliver the viral genome into the host cell.
Sahin and colleagues say this membrane fusion can be blocked by mutating Spike residues 986 and 987 into prolines to form a Spike antigen that is stabilized in the prefusion conformation (P2 S).
The RBD is the main target of neutralizing antibodies following infection with SARS-CoV-2 and has an “up conformation” where many neutralizing epitopes are exposed and a “down” formation” where many epitopes are not exposed.
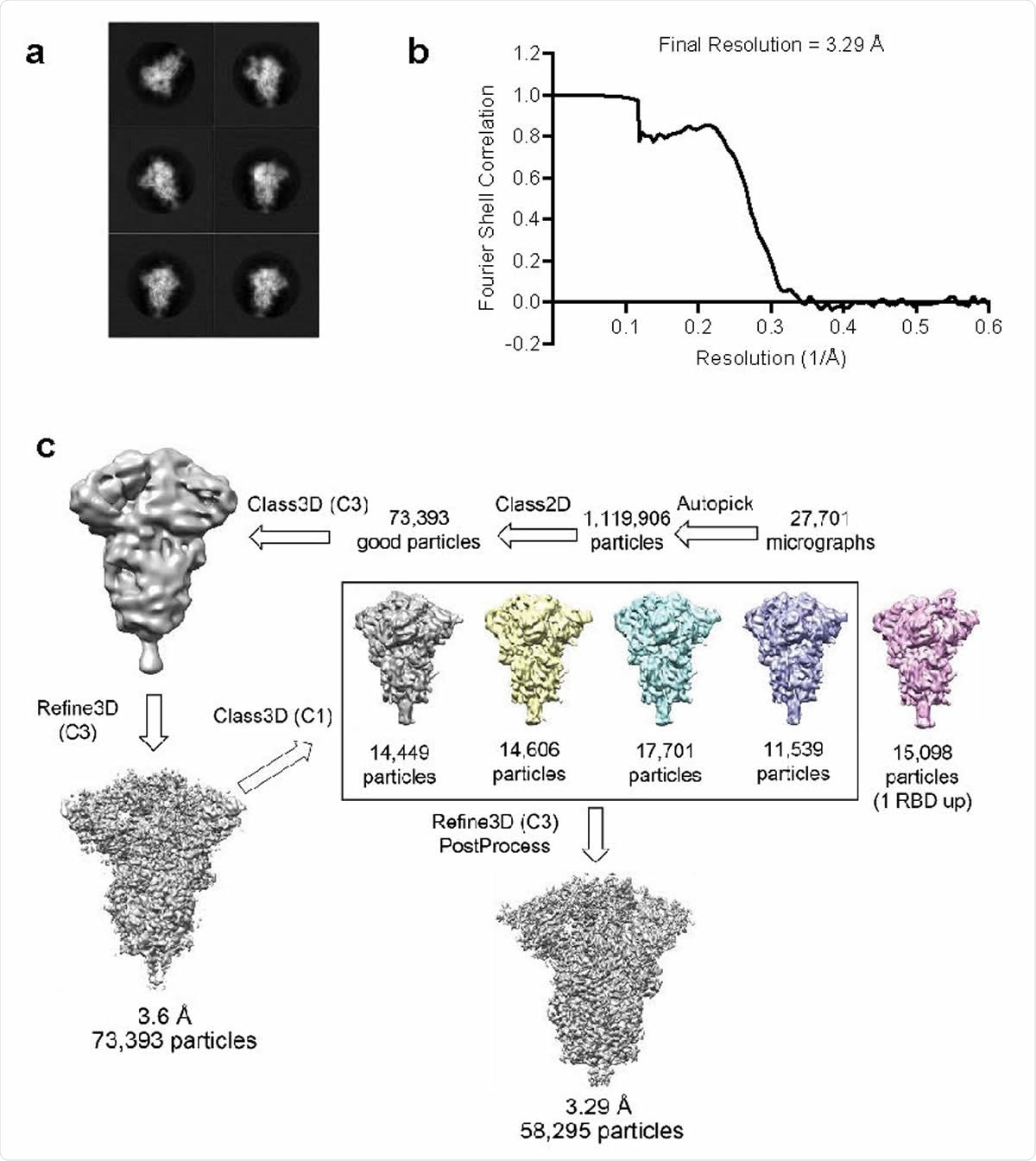
Structure analysis of BNT162b2-encoded P2 S by cryo-electron microscopy a, 2D class averages of TwinStrep-tagged P2 S particles extracted from cryo-EM micrographs.. Box edge: 39.2 nm in each dimension. b, Fourier shell correlation curve from RELION gold-standard refinement of the P2 S trimer. c, Flowchart for cryo-EM data processing of the complex.
What did the current study involve?
The team has described the development of BNT162b2, which contains a nucleoside-modified mRNA that codes for P2 S with a native furin cleavage site, thereby resulting in the S1 and S2 cleavage fragments.
The nucleoside modification dampens innate immune sensing and increases RNA translation in vivo. Previous studies have already shown modified RNA vaccines to be immunogenic against a number of viruses.
Now, Sahin and colleagues have shown that BNT162b2, which encodes the Spike protein captured in a prefusion conformation, is highly immunogenic in mice and rhesus macaques.
After expressing the BNT162b2 coding sequence in cells, about one-fifth of Spike proteins were in the one-RBD ‘up,’ two-RBD ‘down’ state.
“This analysis confirmed that the antigenically important RBD could assume the ‘up’ conformation, with the receptor binding site, rich in neutralizing epitopes, accessible in a proportion of the molecules,” writes the team.
What was the effect of vaccination?
In mice, a single dose of BNT162b2 generated high titers of neutralizing antibodies and potent T-helper 1(TH1) and T follicular helper cells (TFH) type CD4+ T cell responses. It also produced strong IFNγ+, interleukin 2 (IL-2+), and CD8+ T-cell responses.
“Both BNT162b2 induced CD4+ T-cell types may support antigen-specific antibody generation and maturation, and potentially protection from infectious challenge,” writes the team.
Prime-boost vaccination in rhesus macaques generated titers of neutralizing antibodies that were between 10.2 and 18.0 times higher than those seen when a SARS-CoV-2 convalescent human serum panel was used. Furthermore, the neutralizing antibody titer was still 3.3 times higher than this benchmark five weeks later.
In addition, the vaccine fully protected the lungs of rhesus macaques (aged 2 to 4 years) from infection, following the SARS-CoV-2 challenge. Testing by reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR) found no detectable SARS-CoV-2 RNA in serial samples of bronchoalveolar lavage taken from the animals 3 days post-challenge.
The authors say the potent TH1-biased CD4+ T-cell response and IFNγ+ CD8+ T-cell response to BNT162b2 are desirable for vaccine safety and efficacy studies and provide reassurance for clinical translation.
“A global, pivotal, phase 2/3 safety and efficacy trial of immunization with BNT162b2 (NCT04368728) is now well underway,” says the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Source:
Journal references: