Pandemics caused by novel infectious agents like the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Zika, or Ebola virus seriously impact human health and lives while also overwhelming health care systems all over the world. Early detection and diagnosis allow isolation of the infected patients and contact tracing, which can mitigate the spread of the virus and save lives, especially when a significant number of the patients are asymptomatic.
Developing low-cost and rapid testing methods for early detection of infection has always been a challenge while dealing with the current COVID-19 pandemic. Presently, two approaches are being used for the detection of COVID-19 infection.
The first one detects the viral genome and uses techniques such as genomic sequencing, CRISPR, and reverse transcription real-time quantitative polymerase chain reaction (PCR). While these methods are relatively accurate, they are slow because there are multiple steps involved in sample processing, and the samples need to be shipped to a laboratory for testing. The accuracy of these methods is also affected by the high mutation rate in the viral genome.
A second approach involves the detection of antibodies specific to the viral antigens using serological methods such as lateral flow immunoassay and enzyme-linked immunosorbent assay (ELISA). These assays have a long sample preparation time (0.5-2 hrs) and are based on structural proteins like the nucleocapsid protein (N-protein) or the spike protein (S-protein). Their sensitivity is relatively low, and false-positive rates are about 5-11%.
The shortfalls of both testing methods show that there is an urgent need for the development of a faster, more sensitive, and specific assay for the detection of SARS-CoV-2 infection.
The authors say, “Hence, there is an urgent need to develop a sensitive and specific assay for rapid detection of SARS-CoV-2 infection within minutes or if possible, seconds; ideally within days of infection, which could particularly be useful for medically underserved areas if a smartphone enables the readout.”
A New Rapid Testing Platform
In a paper published on the preprint server medRxiv*, researchers from the Carnegie Mellon University and University of Pittsburgh School of Medicine discuss a newly developed 3D printed biosensing platform called the 3DcC that can sense SARS-CoV-2 antibodies within seconds.
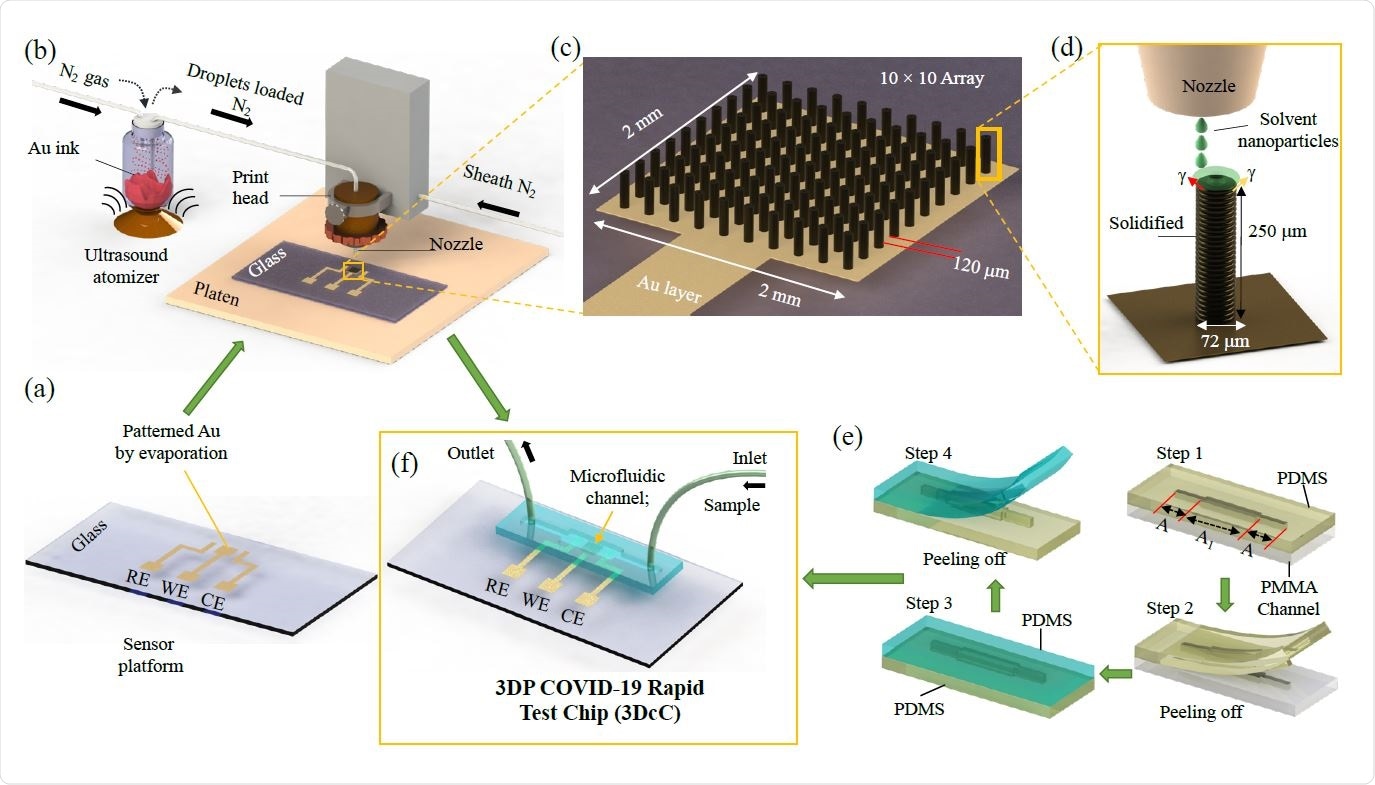
Schematic of the Manufacturing process of the 3D Printed COVID-19 Test Chip (3DcC) by Aerosol Jet nanoparticle 3D printing

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The platform was developed by using aerosol jet nanoparticle 3D printing method to produce gold micropillar array electrodes, which was followed by functionalization of rGO and antigen immobilization on the electrode surface with the help of an EDC: NHS chemistry.
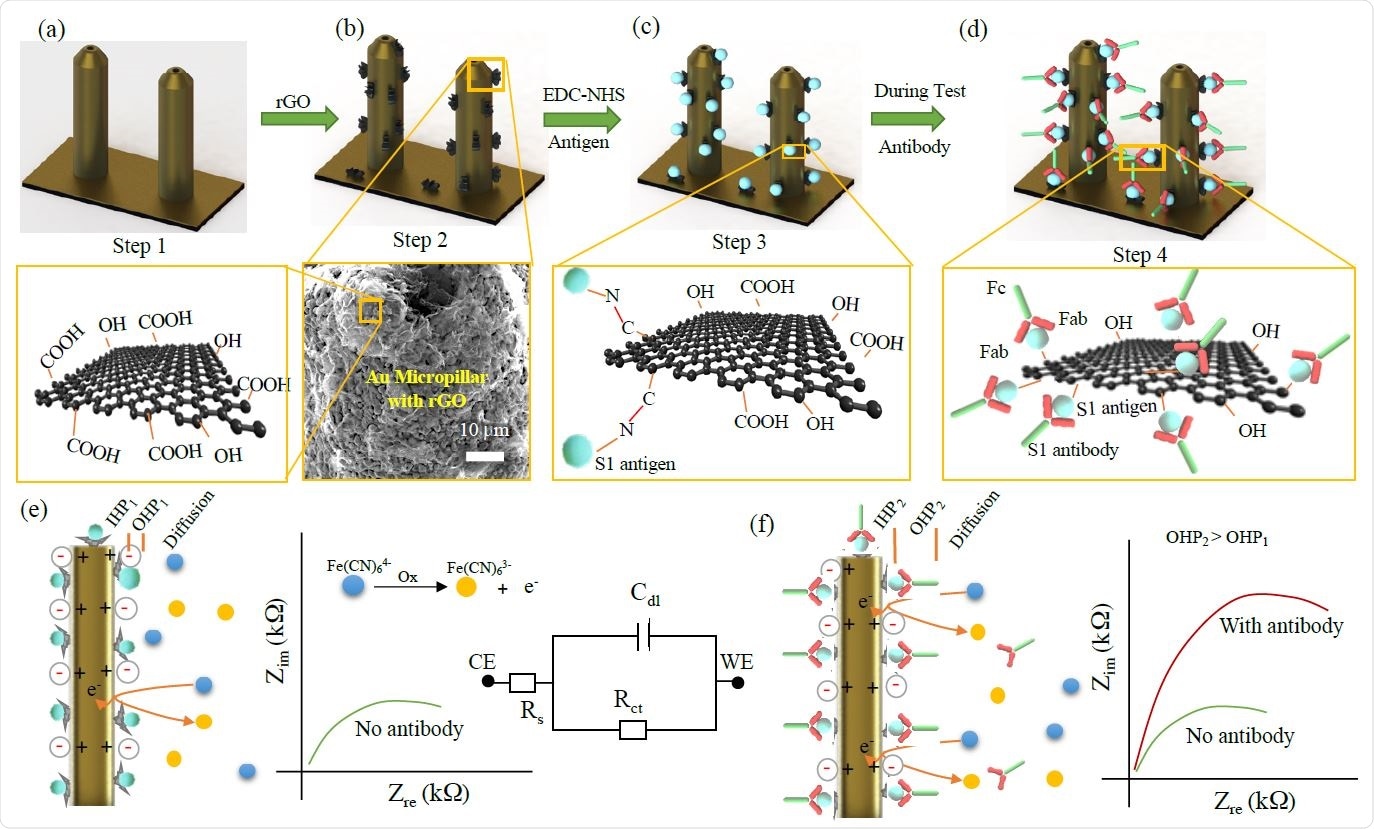
Functionalization of 3D Printed Micropillar Electrode and 3DcC Sensor Operation.
The device is capable of detecting antibodies produced against the S1 protein and receptor-binding-domain (RBD) of SARS-CoV-2 at very low concentrations (1pM) using electrochemical impedance spectroscopy. The results can be read on a smartphone-based user interface. Moreover, the sensor used in the device can be regenerated in under a minute at a low-pH that elutes the antigens from the antibodies. This allows using the same sensor to test multiple samples. In this work, two antibodies were tested within 11.5 seconds.
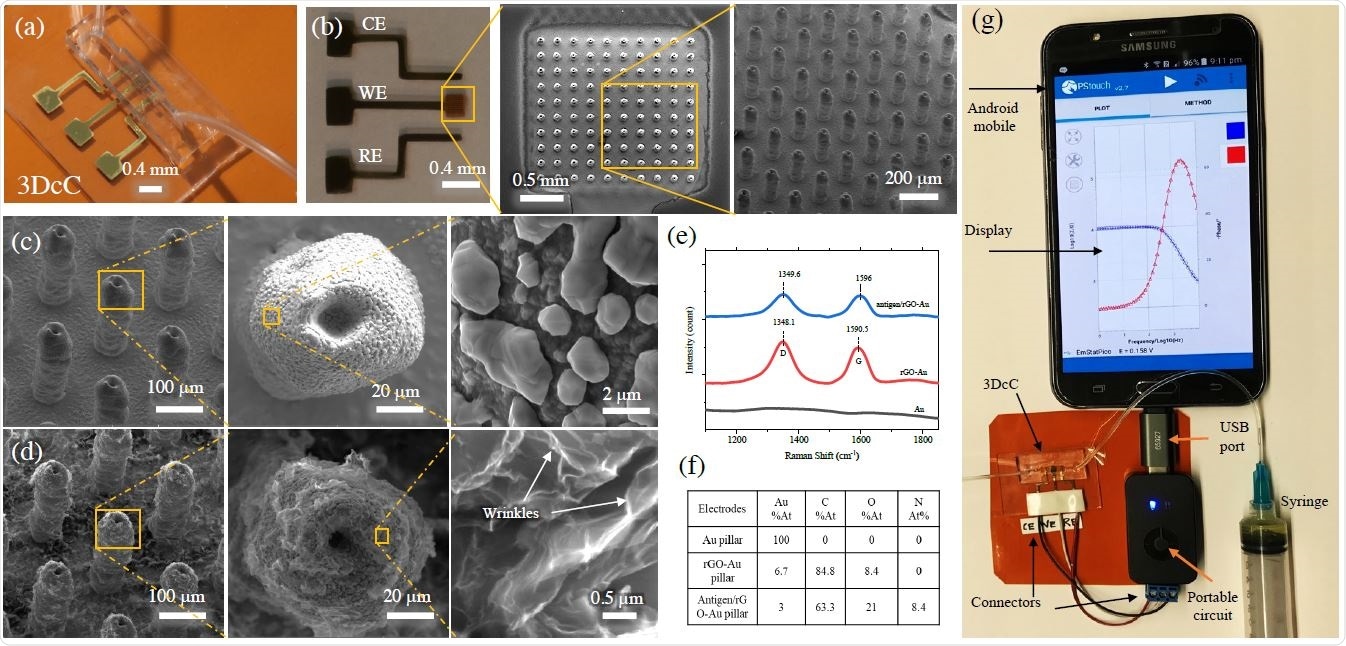
Physical and Chemical Characterization of the 3DcC device
Significance of this biosensing platform
In electrochemical sensing, the antibody-antigen complex is detected through electrochemical transduction. The specificity, sensitivity, and speed of detection depend on the electrochemical cell, the surface chemistry of the electrodes, the antigen, and the type of assay.
This work aimed at utilizing advanced techniques such as nanoparticle 3D printing for microelectronic fabrication and using electrochemical transduction in the rapid detection of COVID-19 antibodies. Combined with a regeneration capability and smartphone-supported design, the new platform offers a rapid, hi-tech, and much-needed solution to early diagnosis of COVID-19.
The signal obtained using this device was very selective and repeatable. Antibodies produced against spike S1 and RBD viral antigens were detected at concentrations as low as 1 pM and 1 fM, respectively. The regeneration of the sensor in less than a minute allowed up to 10 successive, accurate readings using the same sensor.
“These results are highly encouraging and a device for field use, after appropriate human trials, will have a significant positive impact on public health and the course of the current as well as future pandemics.”, write the authors.
This biosensing system will make rapid detection and early diagnosis possible, thus saving lives. Moreover, as this platform is generic, it can find more uses in detecting biomarkers specific to a variety of other pathogens such as HIV, Ebola, and Zika, which makes it a significant advancement in biotechnology that has a very positive impact on public health.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Sensing of COVID-19 Antibodies in Seconds via Aerosol Jet Printed Three Dimensional Electrodes Md Azahar Ali, Chunshan Hu, Sanjida Jahan, Bin Yuan, Mohammad Sadeq Saleh, Enguo Ju, Shou-Jiang Gao, Rahul P Panat medRxiv 2020.09.13.20193722; doi: https://doi.org/10.1101/2020.09.13.20193722
- Peer reviewed and published scientific report.
Ali, Md. Azahar, Chunshan Hu, Sanjida Jahan, Bin Yuan, Mohammad Sadeq Saleh, Enguo Ju, Shou‐Jiang Gao, and Rahul Panat. 2020. “Sensing of COVID‐19 Antibodies in Seconds via Aerosol Jet Nanoprinted Reduced‐Graphene‐Oxide‐Coated 3D Electrodes.” Advanced Materials 33 (7): 2006647. https://doi.org/10.1002/adma.202006647. https://onlinelibrary.wiley.com/doi/10.1002/adma.202006647.