The high burden of disease caused by the COVID-19 pandemic has led to much research on effective ways to mitigate the health, social, and economic impact of the spread of the infection. A recent study published on the preprint server bioRxiv* in September 2020 reports on the characteristics of the drug baricitinib when used as monotherapy to treat severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
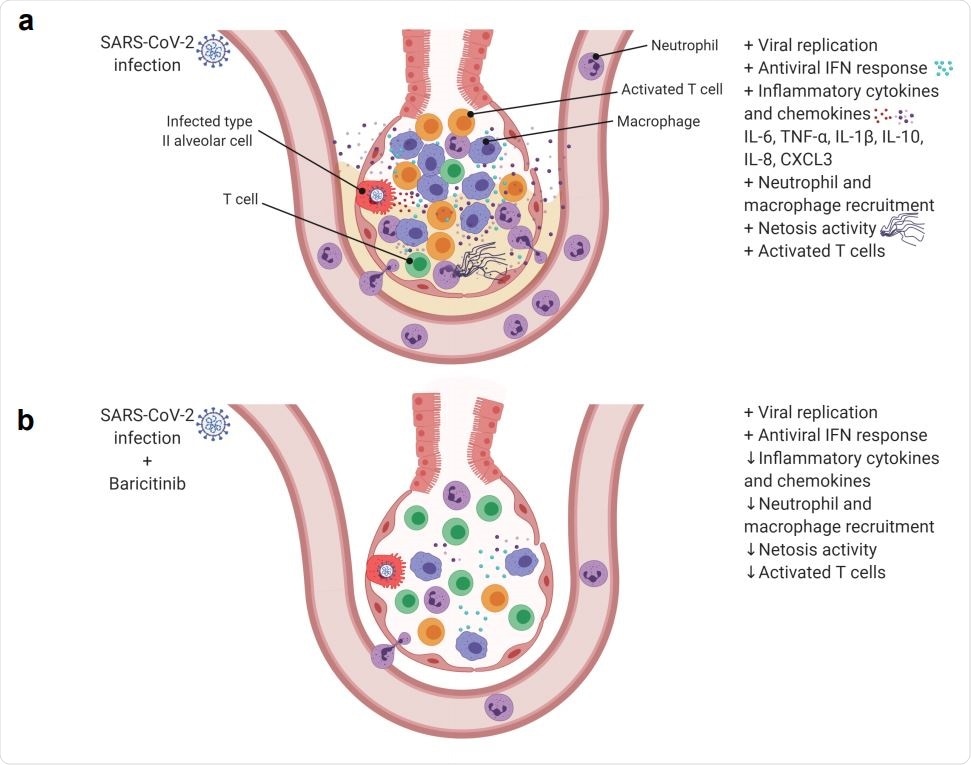
SARS-Cov-2 infection in rhesus macaques results in an accumulation of inflammatory macrophages and neutrophils in the lower airway. These airway macrophages produce high amounts of inflammatory cytokines and neutrophil-attracting chemokines and show upregulated Type I interferon signaling. Neutrophil NETs and the inflammation induced by SARSCoV-2 infection both contribute to lung pathology. (b) Baricitinib treatment reduced the levels of macrophages producing inflammatory cytokines and neutrophil-attracting chemokines, decreased the infiltration of neutrophils into the lung and reduced T cell activation. The Netosis activity of neutrophils was also reduced. In treated animals, the antiviral interferon response was maintained, viral replication was not impacted, and lung pathology was mild.
Severe COVID-19 is associated with high fever, non-productive cough, and pneumonia, with X-rays or CT scans of the chest showing bilateral opacities and evidence of lung inflammation. The infiltration of neutrophils and other inflammatory cells into the lung tissue is a characteristic of progressive disease in this syndrome, with immunocompromised individuals, as well as those with other chronic illnesses, being at higher risk for severe and critical disease.
The hyperactive immune response underlying progressive COVID-19 involves both innate and adaptive immunity. Pro-inflammatory cytokines and chemokines such as IFNγ, TNFα, IP-10, G-CSF, IL-2, IL-6 IL-8, IL-9, IL-10, and IL-17 are observed to be at high levels, which has promoted the use of Janus Kinase inhibitors. These drugs inhibit cytokine release via these pathways and may thus reduce the odds of a cytokine storm and subsequent multi-organ damage.
Baricitinib in NHPs
The current study by researchers at Emory University, Oregon Health & Science University, a Jolla Institute For Allergy & Immunology and Case Western Reserve University uses the non-human primate (NHP) rhesus monkey model to study the role of the selective oral Janus kinase inhibitor baricitinib because they have established that this animal model recapitulates mild to moderate infection with SARS-CoV-2. It is already in use for patients with moderate to severe rheumatoid arthritis.
Its potential for use in COVID-19 was suggested by machine learning algorithms using in vitro data that indicated the possibility that baricitinib might also suppress the endocytosis of this virus via clathrin-mediated pathways. If this is true, the dual action of baricitinib in reducing the intensity of the inflammatory response and in preventing viral entry into the host cell and viral replication might be beneficial.
Baricitinib Does Not Prevent Viral Replication
The current study, therefore, used a longitudinal approach to test tissue responses to baricitinib, using both bronchoalveolar lavage (BAL) and lung tissue samples. They found that it was well-tolerated and achieved therapeutic concentrations in both plasma and in tissue. However, it did not arrest viral replication in infected rhesus monkeys.
Baricitinib Reduces Inflammation
The infected monkeys did show lower severity of lung disease and inflammation and lower levels of cytokines and chemokines involved in the inflammatory pathway. The chief findings of the investigators included the dampening of the genes associated with inflammation and neutrophil activation in BAL of infected monkeys.
DEGs Reduced with Baricitinib
Bulk RNA-Seq profiling of cells obtained from the BAL five days before virus inoculation, two days after inoculation, four days after infection, and 2 days after initiating baricitinib. They found that differentially expressed genes (DEGs) were strongly upregulated two days after infection in both treated and untreated animals. Two days after treatment, however, there was an abrupt drop in DEGs, which was quite the opposite of the robust increase in expression that persisted in the untreated group.
In the treated group, the gene expression at 2 and 4 days from infection was found to show that dampening started at an earlier time point. Many of the downregulated genes encoded enzymes involved in degradation and bactericidal processes, within neutrophil granules, or genes that are expressed at high levels on non-lymphocyte white cells, genes that encode matrix-degrading enzymes during the passage of neutrophils out of the blood vessel, and the alarmin S100A12.
The difference was striking: these genes were expressed at high levels in BAL of untreated animals but at much lower levels following baricitinib treatment. In fact, in the latter group, the levels closely resembled that at baseline.
Thus, neutrophil recruitment and activity in the lower airway resulting from acute infection with the virus is dampened by baricitinib. This is more significant in that many of these genes are highly expressed in myeloid blood cells in severely ill COVID-19 patients.
Baricitinib Suppresses Inflammatory Pathways
Baricitinib also rapidly suppressed inflammatory mediators in the TNFα signaling and IL6 signaling pathways, including factors that induce neutrophil chemotaxis and recruitment of both neutrophils and macrophages. Inflammatory and immune-regulatory cytokines also undergo suppression. These changes are also reflected in the rheumatoid arthritis pathway, as expected from a drug that was developed to reduce signaling in the Janus kinase pathway. In fact, it downregulates the IL-6/JAK/STAT3 signaling pathway of inflammation, as shown above.
Baricitinib does not affect the genes involved in Type I interferon signaling pathways, and interferon-stimulated genes (ISGs), part of the innate antiviral immune response. These genes continued to be at an elevated expression in both treated and untreated groups.
Baricitinib almost completely inhibits the expression of inflammatory cytokines such as TNFa, IL10, IFNb, and IL6, and of neutrophil chemotactic factors in the lung macrophages of the infected monkeys, within just 48 hours of treatment. These cytokines are thought to be part of the disease process in COVID-19. Again, ISG expression was left mainly unchanged even as baricitinib reduces airway inflammation and neutrophil infiltration robustly.
Baricitinib Inhibits Neutrophil Accumulation and NETosis
Baricitinib also produces low levels of neutrophils and consequent NETosis in the BAL. NETs (neutrophil extracellular traps) are also thought to be important in the pathogenesis of inflammation and microvascular thrombosis in these patients. In untreated animals, neutrophils are rapidly recruited at four days from infection, while viremia is at its peak. This high level of neutrophil infiltration was sustained even at later time points of infection but was not observed following baricitinib treatment.
Baricitinib also reduced T cell activation in the monkeys following infection. Whereas proliferating memory CD8+ T cells were found to increase markedly and rapidly at 7 and 10 days after infection, all treated animals showed a significant decrease by 4 days from infection.
Baricitinib Preserves Antiviral Immunity
Baricitinib did not impair the ability of animals to respond by peripheral T cell activation to specific SARS-CoV-2 antigens and other non-antigen stimulation (PMA/ionomycin).
Implications for COVID-19 Treatment
The researchers found that baricitinib reduced lung pathology, inflammatory markers and cytokines, and levels of systemic inflammation in monkeys. All of these are associated with severe COVID-19 disease in humans. At the same time, it did not reduce type 1 IFN responses. This was validated by observing reduced neutrophil infiltration into the lungs and deficient levels of T cell activation in peripheral blood and in the BAL after treatment.
They also found that NETosis was increased in animals after SARS-CoV-2 infection, but reduced after treatment with baricitinib. At the cellular level, single-cell RNA seq methods showed a steep decline in immune activation markers and neutrophil recruitment and macrophage trafficking profiles, in the treatment group.
The findings that high levels of inflammatory cytokines in the lung are linked to severe or critical COVID-19 reflects very recent studies and indicates the clinical potential for baricitinib in dampening the hyperactive immune response that is commonly seen in moderate to severe COVID-19. In fact, it could be superior to drug inhibitors that target specific cytokines, like the IL-6R inhibitor tocilizumab, since it suppresses multiple inflammatory cytokines to prevent a cytokine storm effectively.
Baricitinib also achieved satisfactory levels in lung and central nervous system tissue, without any signs of toxicity. However, it did not suppress viral replication in infected animals. On the other hand, the researchers also found that its downstream immune system suppressive effects did not limit adaptive T cell and Type 1 IFN innate immune responses to the virus.
Ongoing trials looking into the effects of baricitinib in infected humans include the Adaptive COVID-19 Treatment Trial (ACTT-2) and the COV-BARRIER trial. The first is studying baricitinib alongside remdesivir, the second baricitinib monotherapy. A small clinical trial that used baricitinib in 20 severe COVID-19 patients indicated that it inhibited immune dysregulation by lowering the plasma levels of IL6, IL1b, and TNFa, leading to a faster recovery.
The authors of the current study sum up, “Our data provides rationale for baricitinib treatment in COVID-19 to be given in a window where blocking immune inflammation would prevent the formation of a cytokine storm without interfering in the initial responses necessary for preventing viral dissemination and persistence.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources