Every year, emerging and re-emerging viruses, such as SARS-CoV-2, MERS-CoV, Zika virus, Ebola virus, influenza A virus, and Rift Valley fever virus surface from natural reservoirs to infect, disable, and kill people. Today, the number of people infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to rise, with 30.9 million recorded infections and a global death toll nearing 1 million.
The high expense and delay associated with drug development is a significant deterrent to drug research and has prompted drug repurposing instead. Drug repurposing, also called drug repositioning, is a strategy for generating additional value from an existing drug by targeting diseases other than that for which it was initially intended.
A recent study published on the preprint server bioRxiv* in September 2020 shows the value of drug combinations to enhance the therapeutic and preventive efficacy of such repositioned drugs in standard antiviral therapy.
Why Drug Repurposing?
The advantages of drug repurposing include having data on all the manufacturing processes, safety profile, and pharmacological characteristics of existing drugs that have undergone both preclinical and clinical assessments. This ensures that these drugs are much more likely to be successfully marketed than new entries into the drug and vaccine market at lower costs and in a shorter period of time.
A big downside is that monotherapy, the use of a single drug to treat a disease or condition, often induces viral resistance, which is why drug combinations are being sought. Using multiple drugs can reduce the chances of drug resistance because each drug uses a different mechanism to inhibit the viral activity. Such mechanisms can also be synergistic or additive, which allows the doses of individual drugs to be lowered without impairing drug efficacy.
This, in turn, increases the safety margin and reduces the odds of adverse reactions. Moreover, if multiple drugs are used, the chances of their being active against more than one type of virus are higher, and such combinations can be used to fight newly emerging or re-emerging viruses.
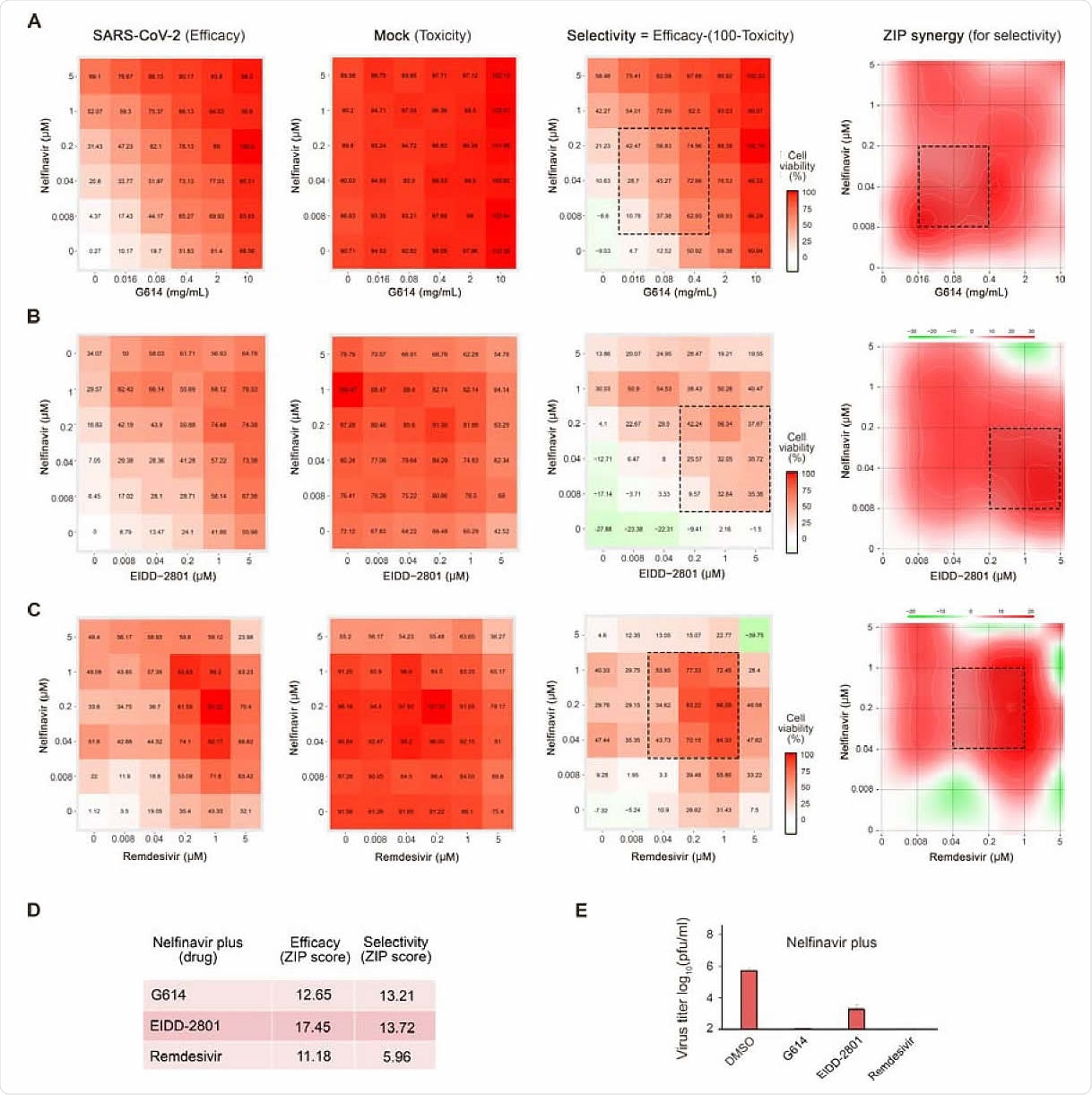
Combinations of nelfinavir with neutralizing antibody, EIDD-2801, or remdesivir rescue Calu-3 cells from SARS-CoV-2-mediated death and inhibit virus replication. (A-C) The interaction drug combination landscapes measured as 6x6 dose-response matrices using a CTG assay on SARS-CoV-2- and mock-infected cells (two left panels). The interaction landscape shows selectivity (efficacy-toxicity) and synergy for the drug combinations (right panels). (D) ZIP synergy scores calculated for efficacy and selectivity dose-response matrices for 3 drug combinations. (E) The effects of 1 μM nelfinavir plus 0,1% DMSO, 2 mg/mL G614, 1 μM EIDD-2801, or 1 μM remdesivir on viral replication measured by plaque reduction assay.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Nelfinavir and Other Antivirals
One such antiviral cocktail is the combination of nelfinavir and remdesivir, which is considered to have the potential to treat 18 or more human viruses. This drug combo is the first choice of treatment of viruses that are changing rapidly.
Many other drug combinations are now in clinical trials against SARS-CoV-2, after showing synergistic activity in vitro when tested against other viruses. Earlier studies showed that when nelfinavir is combined with salinomycin, amodiaquine, obatoclax, emetine, or homoharringtonine, it had synergistic activity against the virus in Vero E6 cells.
The current study reports a combination of nelfinavir, an oral betacoronavirus protease inhibitor, with the investigational drug EIDD-2801 and convalescent serum that proved to have synergism against infection of the human lung epithelial cell line Calu-3. Earlier research by this team indicated that convalescent serum could inhibit viral entry and replications and prevent cell death due to viral infection.
Here they combined the use of convalescent serum with nelfinavir, finding it to achieve synergism against SARS-CoV-2 but without evidence of toxicity. They also confirmed that EIDD-2801 had anti-SARS-CoV-2 activity on Vero cells. Even though they also tested remdesivir and two other drugs reported to have antiviral activity combined with nelfinavir, they were able to confirm synergistic inhibition of SARS-CoV-2 infection only with nelfinavir plus EIDD-2801 or remdesivir. However, the former had a synergy score of 14 vs. 6 for the second combination.
These combinations of nelfinavir with convalescent serum, EIDD-2801, or remdesivir brought down viral titers by 2 or more logs than the use of nelfinavir alone. Nelfinavir was observed to inhibit the SARS-CoV-2 protease, allowing it to be rapidly tested for COVID-19 treatment.
EIDD-2801 was initially developed for the treatment of influenza. Remdesivir has been used in numerous COVID-19 protocols already.
The researchers say, “Further research on combinations of convalescent plasma or neutralizing antibodies such as BD-368-2 with nelfinavir or other viral inhibitors is warranted. We have also uncovered synergism between nelfinavir and EIDD-2801.” These findings will be tested in clinical trials for validation of clinical benefit.
Novel Anti-EV1 and other Antiviral Combinations
The researchers identified a highly effective combination of antivirals targeting EV1, an enterovirus that belongs to a genus of many common human pathogenic viruses. A screen of the FIMM oncology drug library brought up vemurafenib as an inhibitor of EV1 replication. This drug is marketed as an inhibitor of the cellular V600E B-Raf enzyme in advanced melanoma treatment. The assay was, however, carried out in two cell types lacking this B-Raf mutation but exposed to EV1.
This showed that EV1 was inhibited in the cell type A549 (but not RPE) by vemurafenib, which failed to inhibit EV6, showing a specific antiviral effect. Multiple metabolic effects of the drug were identified as well as on transcription factors required for antiviral gene expression. Again, EV1 replication inhibited the secretion of many growth factors, which was reversed by vemurafenib.
This led them to explore combinations of this drug with other safe drugs, which showed that vemurafenib plus emetine, homoharringtonine, gemcitabine, or obatoclax were synergistic (synergy score > 5). On the other hand, a reduction of EV1 replication by 2 logs or more was observed only with vemurafenib combined with either emetin or homoharringtonine.
These two compounds are already thought to be safe in humans, and these combinations should be further developed for novel anti-EV1 therapy.
Other Antiviral Cocktails
Other effective combinations detected in the current study include a five-drug combination targeting echovirus 1, sofosbuvir with brequinar and niclosamide against HCV infection, and monensin in combination with lamivudine and tenofovir against HIV-1 infection.
Making Knowledge Accessible
The investigators also set up a website that will present the complete clinical and scientific information on existing and newly tested combinations of antiviral drugs with synergistic activity. This is to counter the lack of awareness of drug interactions already established through research. They aim to help build a central resource for all such beneficial and adverse interactions between antiviral drugs.
Future Directions
Regarding the future, the investigators say they want to “find potential biomarkers for the prediction of novel drug combinations for the treatment of emerging and re-emerging viral infections” in order to identify effective and safe combinations more rapidly and inexpensively in the future.
Source

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources