Researchers from the NHS Foundation Trust, University of Cambridge and Cambridge Clinical Laboratories have warned that until a vaccine for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) becomes available, antigen and antibody testing should be carried out among oncology nurses as part of routine patient care.
The team’s study of 434 patient-facing oncology staff who worked during the peak of the coronavirus disease 2019 (COVD-19) pandemic in the UK, found that the highest seroprevalence rate for SARS-CoV-2 was among nurses.
David Favara and colleagues say that current UK guidelines recommend that all patients receiving systemic anticancer therapy should be tested for SARS-CoV-2 by PCR (polymerase chain reaction) before starting treatment, with further testing considered at intervals during treatment.
The guidance regarding healthcare workers, on the other hand, only recommends testing in the broadest sense, says the team.
“We propose that there should be a focus on routinely testing oncology nursing staff for both SARS-CoV-2 antigen and antibodies until an effective vaccine comes available,” write the researchers.
A pre-print version of the paper is available on the server medRxiv*, while the article undergoes peer review.
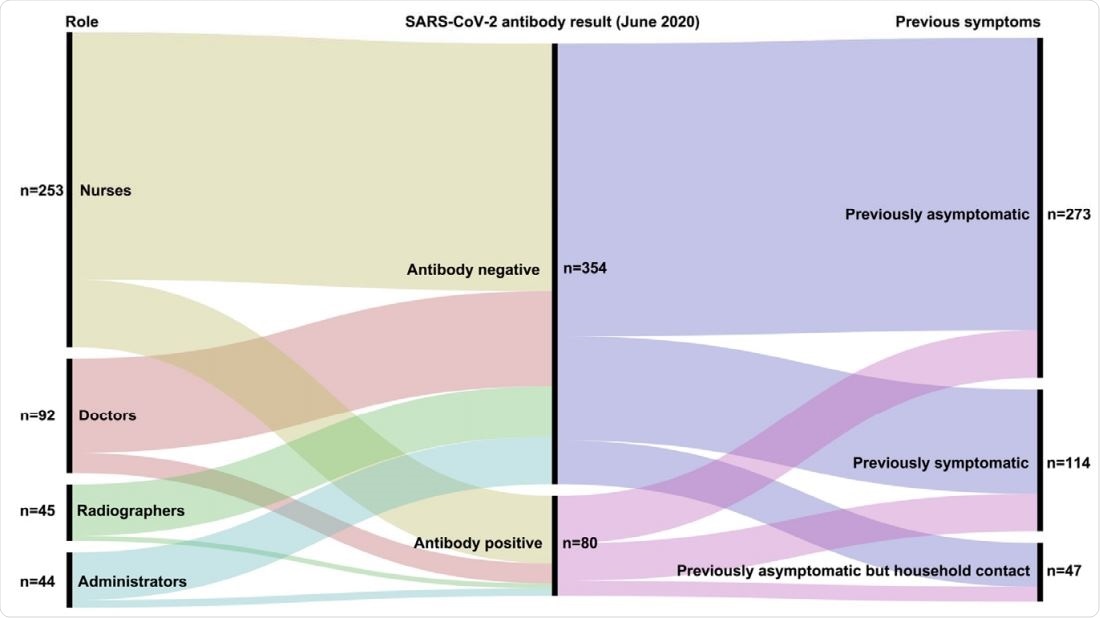
Summary of relationship between role, previous symptoms and antibody results (June 2020 sample collection). All participants were nasopharyngeal swab SARSCOV-2 PCR negative at time of SARS-COV-2 antibody testing.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Cancer patients may be at greater risk of contracting SARS-CoV-2
Since the first cases of SARS-CoV-2 were first identified in Wuhan, China, late last year, the virus has now infected more than 31 million people globally and caused more than 961,000 deaths. Despite researchers’ intense efforts to develop therapies, no effective antiviral treatments or vaccines have yet been developed.
Cancer patients may be at a greater risk of contracting SARS-CoV-2 owing to the multiple hospital visits they need to attend for diagnosis, treatment, and follow-up.
Recent studies have suggested that while anticancer therapy does not increase the mortality risk among cancer patients infected with SARS-CoV-2, it may increase the risk of severe complications following infection.
Guidance is therefore needed to safeguard both patients and oncology staff, say Favara and colleagues.
However, data regarding oncology-specific SARS-CoV-2 infection and immunity rates in the UK are lacking, and the risk of transmission among staff who care for cancer patients is not known.
“To date, no large study has specifically reported and tracked patient-facing oncology staff SARS-CoV-2 exposure,” say Favara and team.
What did the current study involve?
Favara and colleagues recruited 434 patient-facing oncology staff who worked during the peak of the COVID-19 pandemic at three secondary care NHS Foundation Trust hospitals in the UK, namely the Queen Elizabeth Hospital, Peterborough City Hospital, and Cambridge University Hospitals.
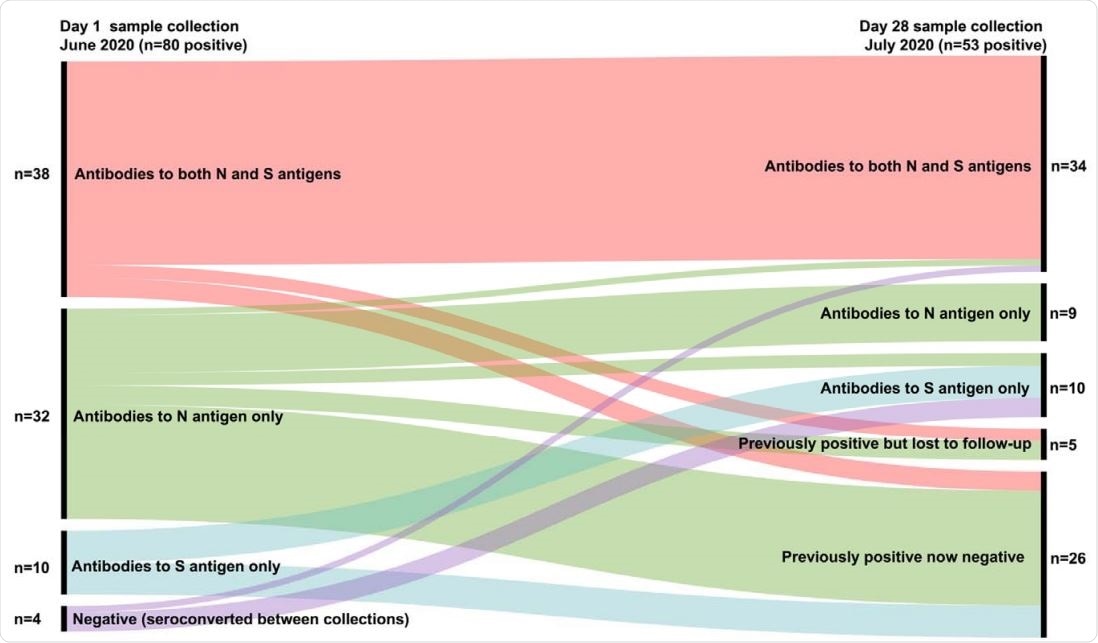
Summary of Relationship between day 1 and day 28 positive antibody results (by SARS-CoV-2 antigen target).
Staff members had nasopharyngeal swabs tested for SARS-CoV-2 by PCR in June 2020 (day 1 samples) and again in July (day 28 samples). They also had their blood tested for SARS-CoV-2-specific antibodies (at the same points) using a laboratory Luminex-based assay and a rapid point-of-care (POC) assay.
Of the 434 participants involved in the study, 58.3% were nurses, 21.2% were doctors, 10.4% were radiographers, and 10.1% were administrators. The overall median age of the study population was 40 years and 82% were female.
Prior to June, 26.3% of participants reported having symptoms indicating potential SARS-CoV-2 infection, and 1.4% had tested positive for infection by PCR.
What did the study find?
On day 1 and day 28 of testing, all participants tested negative for SARS-CoV-2 by PCR.
The Luminex-based assay identified 18.4% of participants as SARS-CoV-2 seropositive on day 1, 42.5% of whom also tested seropositive by PCO.
Luminex-based seropositivity rates were higher among nurses (21.3%) and doctors (17.4%), compared with among administration staff (13.6%) and radiographers (8.9%).
Of 400 participants who also underwent testing on day 28 in July, 13.3% tested seropositive by Luminex, 92·5% of whom had previously tested positive, and 7·5% of whom were newly positive.
Of all the staff groups tested, the seroprevalence rate was highest among nurses, at 16.5%.
“The daily interactions of nurses with multiple patients at close quarters will undoubtedly contribute to these stark statistics,” say Favara and colleagues.
Of the participants who tested seropositive on day 1, 32.5% became seronegative by day 28, suggesting that SARS-CoV-2 antibody seropositivity declines over time.
Nurses should be tested regularly as part of routine patient care
The researchers say that until a vaccine becomes available, the high prevalence of SARS-CoV-2 seropositivity in oncology nurses, along with the high rate of decline in seropositivity over 4 weeks supports regular antigen and antibody testing in this staff group as part of routine patient care.
“This study sets the first seropositivity baseline for UK oncology staff and provides new information to consider incorporating into international guidance on safeguarding patients,” say Favara and team. “We propose that there should be a focus on routinely testing oncology nursing staff for both SARS-CoV-2 antigen and antibodies until an effective vaccine comes available.”
The researchers suggest that since seropositivity can fluctuate within 4 weeks, testing should be carried out at least once a month. Ideally, weekly PCR-testing with fortnightly serology would be performed.
“Increasing availability of lower-cost, high sensitivity, and specificity SARS-CoV-2 testing methods should make this targeted approach viable, would help protect patients and staff and enable containment and tracking of new, asymptomatic infections,” they conclude.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Favara D, et al. SARS-CoV-2 antigen and antibody prevalence among UK staff working with cancer patients during the COVID-19 pandemic. medRxiv, 2020. doi: https://doi.org/10.1101/2020.09.18.20197590
- Peer reviewed and published scientific report.
Favara, D.M., K. McAdam, A. Cooke, A. Bordessa-Kelly, I. Budriunaite, S. Bossingham, S. Houghton, R. Doffinger, N. Ainsworth, and P.G. Corrie. 2021. “SARS-CoV-2 Infection and Antibody Seroprevalence among UK Healthcare Professionals Working with Cancer Patients during the First Wave of the COVID-19 Pandemic.” Clinical Oncology 33 (10): 667–75. https://doi.org/10.1016/j.clon.2021.04.005. https://www.clinicaloncologyonline.net/article/S0936-6555(21)00154-0/fulltext.