Researchers in the United States and Japan have conducted a study suggesting that a commonly occurring genetic variant influences susceptibility to infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the agent that causes coronavirus disease 2019 (COVID-19).
The team found that allelic conversion at the common variant rs4702 impacted SARS-CoV-2 infection in human neurons and alveolar cells in vitro.
Kristen Brennand (Icahn School of Medicine at Mount Sinai, New York) and colleagues say their study provides a proof-of-principle finding that common human genetic variation may directly impact susceptibility to SARS-CoV-2 infection and therefore contribute to the variability in host responses between individuals.
“This work supports ongoing efforts to discover host genes associated with SARS-CoV-2 infection, both in vitro and in the clinic,” said the researchers. “Our hope is that such efforts might better predict clinical outcomes before the onset of symptoms and facilitate the discovery of drugs that might prevent or treat COVID-19 disease.”
A pre-print version of the paper is available in the server bioRxiv*, while the article undergoes peer review.
Inter-individual variability in host response to SARS-CoV-2
The host response to SARS-CoV-2 infection varies significantly between individuals, with outcomes ranging from asymptomatic infection to mild-to-moderate symptoms and severe or critical disease.
Furthermore, although men, the elderly, and people with underlying health conditions are more likely to develop severe disease, SARS-CoV-2 can also cause severe complications in healthy individuals with none of these risk factors.
The different clinical outcomes following infection do not seem to be simply due to variations in the adaptive immune response since a growing body of evidence suggests that seroconversion can occur before symptoms have resolved.
A number of human genetic variants have previously been identified that influence susceptibility or resistance to infection with viruses, including influenza, respiratory syncytial virus, norovirus, rotavirus, parvovirus, and human immunodeficiency virus.
“We hypothesize that, in addition to viral load and host antibody repertoire, host genetic variants also impact vulnerability to infection,” write Brennand and colleagues.
How does SARS-CoV-2 infect cells?
In order to infect target cells, SARS-CoV-2 uses a surface viral protein called spike to bind the host cell receptor angiotensin-converting enzyme 2 (ACE2) and the host cellular transmembrane protease, serine 2 (TMPRSS2). SARS-CoV-2 fuses to the host cell membrane once the spike protein has been processed by TMPRSS2 to reveal the fusion peptide.
The virus has evolved a four amino acid insertion that is thought to be cleaved by a host membrane-bound proprotease convertase called furin in order to prime the spike protein for TMPRSS2 processing.
“In contrast to SARS-CoV-2, the SARS-CoV spike protein lacks this FURIN-cleavage site, thus requiring cleavage to facilitate subsequent cell entry,” writes Brennand and colleagues. “This theoretical hijacking of host FURIN activity is one possible explanation for the increased infectivity of SARS-CoV-2.”
What did the current study involve?
To determine whether host variants that may influence SARS-CoV-2 host cell entry might contribute to the between-individual variability in COVID-19 symptoms, the researchers assessed variants of the FURIN gene across human induced pluripotent stem cells (hiPSC)-derived lung, intestinal, and brain models.
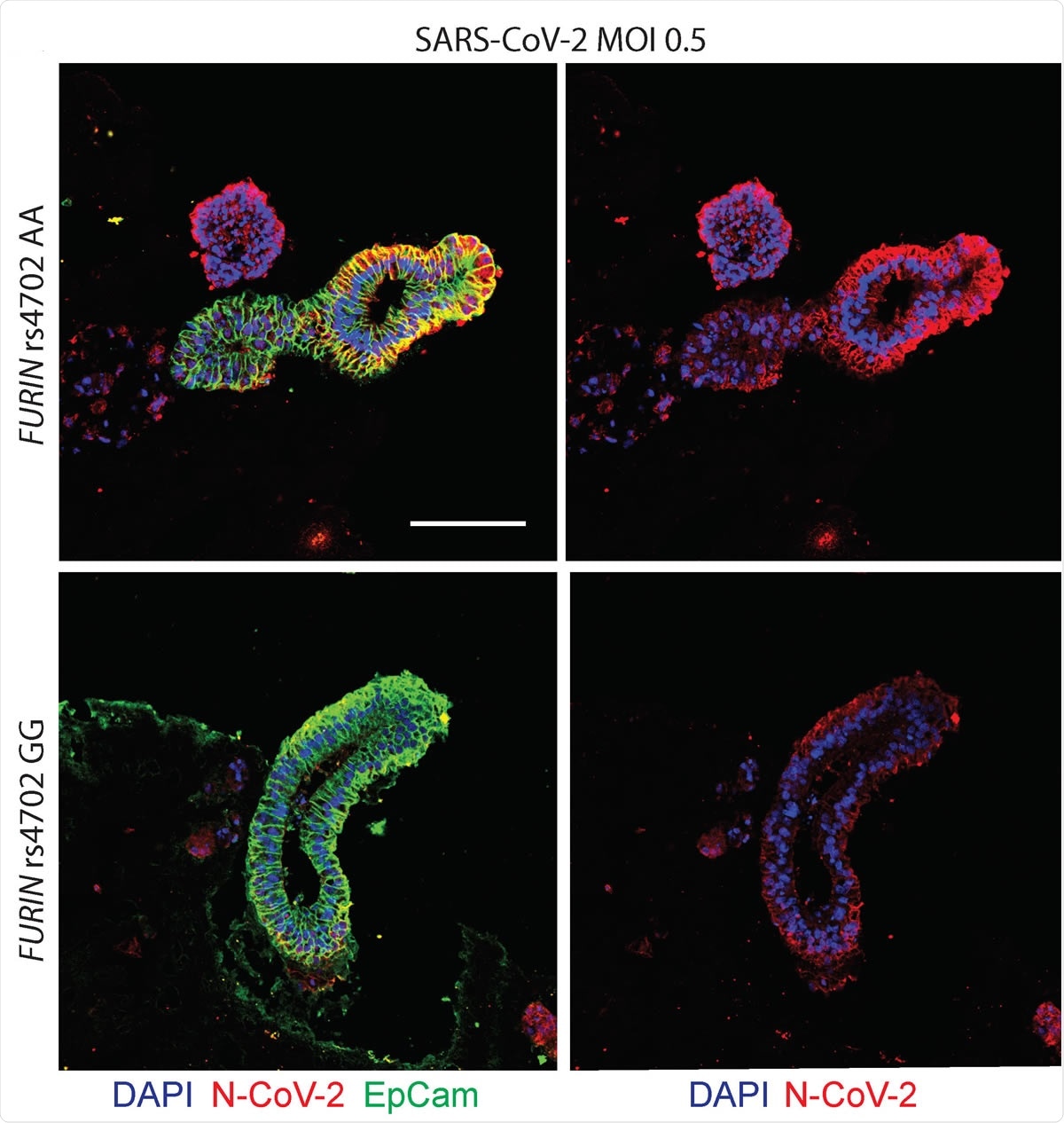
Allelic conversion at FURIN rs4702 in alveolospheres and neurons impacts SARS-CoV-2 infection. Representative immunofluorescence staining against SARS-CoV-2 nucleocapsid (N) protein (red), epithelial marker EPCAM (green), and DAPI (blue). Alveolospheres were generated from C2 FURIN rs4702 AA and GG lines and infected with mock or a MOI of 0.5 SARS-CoV-2 for 24 hours. Scale bar: 100 μm.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The results showed the importance of furin as a mediator for SARS-CoV-2 infection and demonstrated that a commonly occurring variant in the FURIN gene is capable of influencing SARS-CoV-2 infection in vitro.
More specifically, CRISPR/Cas9-mediated allelic conversion of the variant rs4702 from AA to GG resulted in decreased expression of the cis-gene target FURIN in neurons and alveolar cells, and reduced SARS-CoV-2 infection.
What are the implications of the study?
The study findings have shown that a single non-coding SNP (single nucleotide polymorphism) is sufficient to impact SARS-CoV-2 infection in human neurons and alveolar cells, say Brennand and colleagues.
“Thus, we provide a proof-of-principle finding that common genetic variation can impact viral infection, and thus contribute to clinical heterogeneity in SARS-CoV-2,” writes the team.
The researchers say the results suggest that uncovering the genetic underpinnings of SARS-CoV-2 outcomes could help to predict COVID-19 susceptibility, as well as facilitate the development of precision treatment and prevention approaches.
“Ongoing genetic studies will help to better identify high-risk individuals, predict clinical complications, and facilitate the discovery of drugs that might treat disease,” they conclude.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Brennand K, et al. Common genetic variation in humans impacts in vitro susceptibility to SARS-CoV-2 infection. bioRxiv, 2020. doi: https://www.biorxiv.org/content/10.1101/2020.09.20.300574v2
- Peer reviewed and published scientific report.
Dobrindt, Kristina, Daisy A. Hoagland, Carina Seah, Bibi Kassim, Callan P. O’Shea, Aleta Murphy, Marina Iskhakova, et al. 2021. “Common Genetic Variation in Humans Impacts in Vitro Susceptibility to SARS-CoV-2 Infection.” Stem Cell Reports 16 (3): 505–18. https://doi.org/10.1016/j.stemcr.2021.02.010. https://www.cell.com/stem-cell-reports/fulltext/S2213-6711(21)00090-4.