Nine months into the global pandemic, which has infected more than 32 million people, there is still no approved effective and safe drug or vaccine against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Scientists and pharmaceutical firms are scrambling to find a vaccine to neutralize SARS-CoV-2 or effective treatment for coronavirus disease (COVID-19).
There is a new glimmer of hope as scientists at the Virginia Commonwealth University (VCU) Massey Cancer Center have found that an experimental cancer drug called AR-12 helps prevent the SARS-CoV-2 from infecting cells and replicating in the body.
Published in the journal Biochemical Pharmacology, the study highlights how drugs intended for other diseases can help fight against COVID-19, which has now claimed over 980,000 lives worldwide. With the promising results, scientists at VCU are now taking steps to launch a clinical trial to test the drug in their health facility.
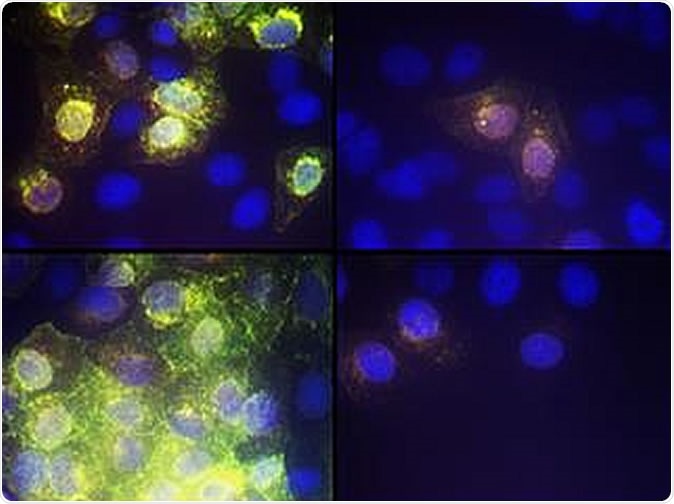
Image showing the effect of AR-12 on SARS-CoV-2 virus replication CREDIT VCU Massey Cancer Center
What is AR-12?
The AR-12 is an orally bioavailable, small-molecule, celecoxib-derive inhibitor of phosphoinositide-dependent kinase-1 (PDK1).
Dr. Paul Dent from the Virginia Commonwealth University and his colleagues have extensively studied AR-12 as both an anti-cancer and anti-viral treatment. In the past, many studies from Dr. Dent showed that the drug is an effective treatment for viral infections, including rubella, chikungunya, Zika virus, mumps, influenza, and drug-resistant human immunodeficiency virus (HIV).
Now, the research team has demonstrated that the drug is also effective against SARS-CoV-2, the virus that causes COVID-19.
"AR-12 works in a unique way. Unlike any other anti-viral drug, it inhibits cellular chaperones, which are proteins that are required to maintain the right 3D shape of viral proteins. The shape of the virus is critical to its ability to infect and replicate," Dent explained.
The AR-12 drug is shown to inhibit one of the cellular chaperones, GRP78, which is vital for the replication of all viruses and acts as a cellular stress sensor. It is needed for the life cycle of all mammalian viruses. Targeting this cellular chaperone can help curb the spread of the novel coronavirus.
Human trials
With the promising new drug, the scientists hope to establish human trials as soon as possible to test the efficacy and safety of the drug on COVID-19 patients. The scientists said that AR-12 therapy is safe and well-tolerated in a past clinical trial.
Further, the AR-12 is not administered intravenously as it is in the form of an oral pill. This makes it unique since it can be used as an outpatient drug.
The team plans to enroll patients in the first quarter of 2021, but there is so much more to do. First, the team needs to formulate a clinical trial protocol and receive a green light from the U.S. Food and Drug Administration (FDA) before proceeding with the trial.
In the meantime, the team is gathering funds to develop enough doses for the drugs to be used in trials. The team formed C19 Therapeutics, which recently licensed AR-12 from VCU to raise funds supporting drug synthesis and clinical trial sponsorship.
"We are working to submit the required information for FDA approvals, and we are also in discussions with a local pharmaceutical company to manufacture the drug for the trial. We are hopeful that AR-12 will emerge as a treatment option for patients suffering from COVID-19, ultimately saving lives and contributing to the global pandemic solution," Said Sebti, associate director for basic research and member of the Developmental Therapeutics program at VCU Massey Cancer Center, said.
Global COVID-19 toll
Finding an effective drug is crucial in the battle against COVID-19, as the virus has reached many countries. The United States has now reported more than 6.97 million people and has killed more than 202,000 people.
Other countries experience the same scenario as their cases continue to skyrocket. India comes in second, with more than 5.73 million infections, while Brazil has more than 4.62 million cases.
In Southeast Asia, the Philippines reported the highest number of infections, with a staggering 296,755 confirmed cases and more than 5,100 deaths.
Source:
Journal reference:
- Rayner, Roberts, Kim, Poklepovic, A, Robets, J., Boith, L., and Dent, P. (2020). AR12 (OSU-03012) suppresses GRP78 expression and inhibits SARS-CoV-2 replication. Biochemical Pharmacology. https://www.sciencedirect.com/science/article/pii/S0006295220304639