Healthcare systems worldwide are overwhelmed by the raging COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As the current number of global infections of SARS-CoV-2 nears 33 million individuals, an effective treatment or vaccine option is crucial and desperately needed.
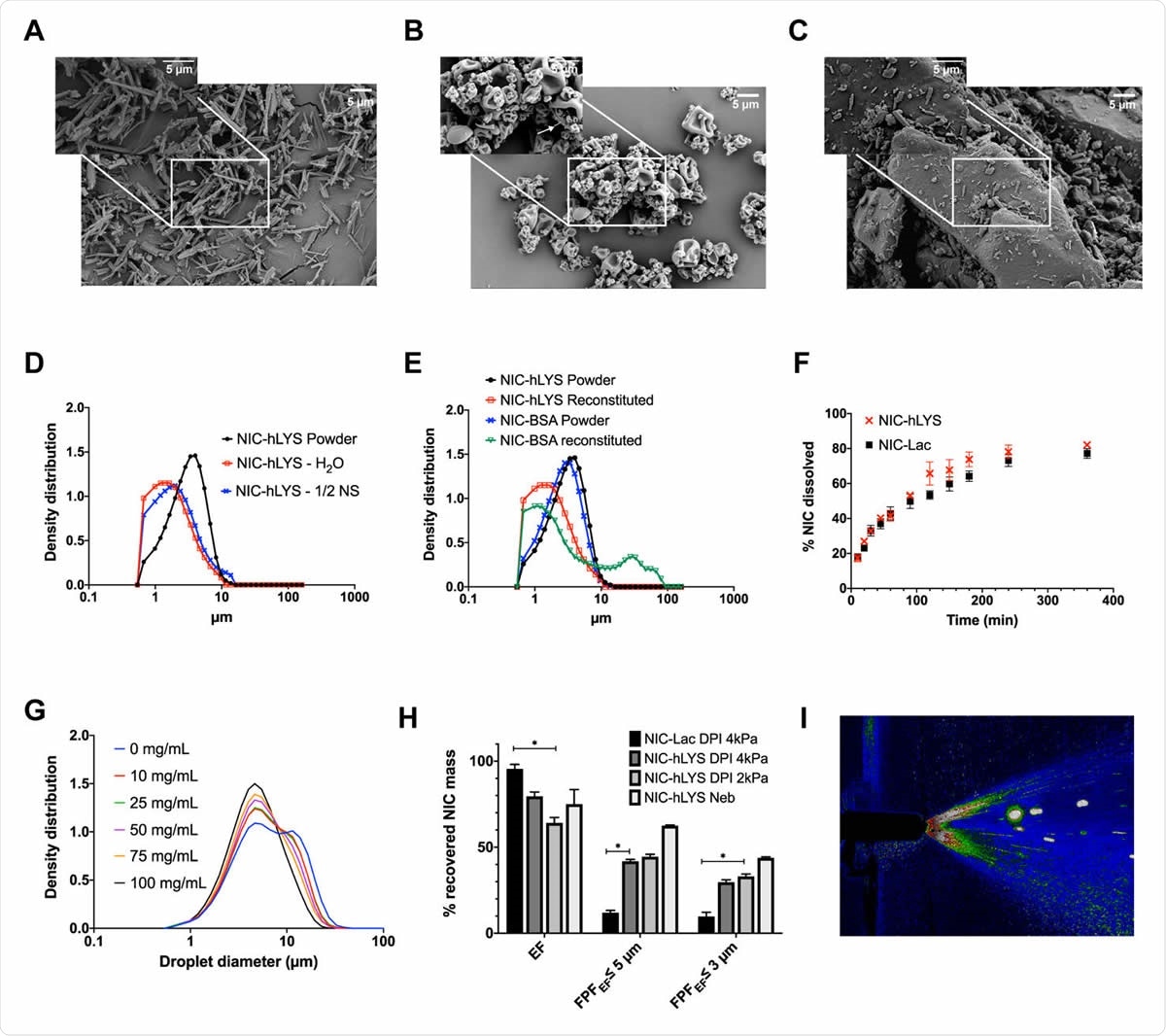
Micronized niclosamide (A) was embedded in a matrix of human lysozyme and stabilizers using spray drying (B). This novel system was developed as an alternative to traditional lactose-based carrier systems (C) and enabled the targeted respiratory delivery of NIC as a powder via DPI or a reconstituted suspension via nebulizer or nasal spray. The optimized formulation exhibited a size distribution that was appropriate for inhalation (i.e., geometric median diameter < 5 μm) in both the dry powder state as well as when reconstituted using water or 0.45% NaCl (D). Similar effects could not be achieved when a negatively charged protein, bovine serum albumin, was substituted in the formulation for the positively charged hLYS (E). Though hLYS is surface active, it appeared to only slightly enhance the dissolution of NIC compared to NIC particles blended with lactose (F). A respirable droplet size distribution could be achieved with multiple different reconstituted concentrations when nebulized using the Aerogen Solo (G). These concentrations resulted in no aggregation to the lysozyme component. Efficient aerosol delivery was achieved with both the nebulizer and disposable DPI, with ~50% of the emitted dose being of an appropriate size for lung deposition. This was significantly improved compared to a traditional lactose carrier particle system (H). Reproducible plume geometry could be achieved using a variety of reconstituted concentrations when actuated using the Aptar device (I). Data is presented as mean + SEM (n = 3). *p < 0.05, using two-way ANOVA with Tukey’s multiple comparisons test (comparisons of DPIs presented only).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
In a strategy to assess and repurpose previously used drugs (against SARS-CoV and MERS-CoV), Ashlee D. Brunaugh et al. choose an FDA-approved anthelmintic (antiparasitic) drug called niclosamide (NIC) to study as a promising antiviral candidate. Niclosamide is listed as an Essential Medicine by WHO and has been in use for over 60 years. In their paper, the authors show the antiviral, antibacterial, and anti-inflammatory efficacy of the NIC-hLYS powders evaluated in vitro and in vivo in MERS-CoV SARS-CoV-2 infected mouse models. Utilizing repurposed NIC with lysozyme, the study reports developing a therapeutically effective, rapidly scalable and globally distributable antiviral therapy to reduce the spread of SARS-CoV-2.
For choosing NIC as a drug in this study, the main properties and observations of actions are:
- NIC has inhibited the MER-CoV replication by modifying pathways in proteosome and autophagy mechanisms.
- The concentration required to inhibit SARS-CoV is low: IC50 of NIC < 0.1 µM and IC50 of chloroquine was 4.4 µM.
- It is active against both gram-positive and gram-negative bacteria, including Staphylococcus aureus, Pseudomonas aeruginosa, Acinetobacter baumannii , Mycobacterium tuberculosis, and bacterial biofilms – all of which may protect against secondary bacterial pneumonia associated with COVID-19.
- NIC inhibits inflammatory cytokine release, protecting against a cytokine storm and acute respiratory distress syndrome (ARDS) – a significant development in severe COVID-19 patients.
However, NIC exhibits poor systemic availability due to its low water solubility (1.6 mg/L). Direct delivery to the lung employing a carrier molecule has been tried before; however, an ideal system was not developed. Keeping in mind rapid administration time, ease of use, and utilization as a prophylactic therapy (especially among high-risk populations such as health workers and first-time responders), the authors adopt three major models or respiratory tract delivery systems: DPI, nasal spray, and nebulizer.
The authors use human-lysozyme (hLYS) as a therapeutically active matrix material for the delivery of NIC to the airways in the lungs. Lysozyme is a protein with anti-inflammatory, antiviral, and antibacterial activity as well as surface-active properties. After processing and aerosolization, the hLYS exhibits stability.
The authors observed the potency of NIC-hLYS against coronavirus in vitro. Compared to NIC alone, the addition of hLYS resulted in improved antiviral activity. After 24 hours of infection, 82.2 % of viral load was decreased in MERS-CoV infection, and 92.7% in SARS-CoV-2 infected cells. They also observed that the highest dose of NIC-hLYS did not affect cell viability when compared with untreated controls. They also observed that hLYS alone also exhibited activity against SARS-CoV-2. Further tests reveal that an improvement in NIC-solubility alone is not responsible for effective antiviral activity. Coupling with hLYS in the formulation has a significant role to play.
The NIC-hLYS was administered via the intranasal route in CoV-infected mice. They observed improved survivability and reduced viral loads in the lungs, brain, and kidneys. The lung tissue in these mice showed lower levels of interstitial pneumonia and reduced inflammation compared to infected and non-treated mice. The surviving animals had also developed specific antibodies against the infection. The NIC-hLYS particles also protect against secondary bacterial infection and inflammatory response; ant-inflammatory activity was also significant.
The authors describe an inhalable NIC formulation. They characterized the optimized powder (NIC-hLYS), using an endogenous protein, and developed and tested for nasal, DPI, and nebulizer systems. They performed testing the delivery with commercially-available devices: a disposable DPI (TwinCaps®), a vibrating mesh nebulizer (Aerogen Solo®), and a nasal spray (VP7 Aptar®). They optimized the composition of the formulation to suit the respective administration method.
The study presents a targeted delivery of the novel possible drug with substantive and broadly-applicable therapy benefits, for the treatment of COVID-19. Observed results and detailed description of the advantages and drawbacks of using each of the devices are provided in the study.
The current FDA approved drug for all hospitalized adult, and pediatric patients with COVID-19 is remdesivir. However, it is not recommended for patients with acute or chronic kidney disease. Based on this study, NIC may be a promising alternative or adjunct therapy. The broad-spectrum activity of NIC-hLYS, shown in the study, may provide a unique advantage over other leading drug candidates against COVID-19. While the authors acknowledge the limitations in their study and the need for further pharmacokinetic evaluation, they report the development of a novel formulation of NIC-hLYS optimized for delivery to the upper and lower respiratory tracts as a powder or stable suspension. They conclude that NIC-hLYS has efficacy in the treatment of the current SARS-CoV-2 infection as well as in future CoV pandemics.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Broad-spectrum, patient-adaptable inhaled niclosamide-lysozyme particles are efficacious against coronaviruses in lethal murine infection models, Ashlee D Brunaugh, Hyojong Seo, Zachary Warnken, Li Ding, Sang Heui Seo, Hugh D.C. Smyth, bioRxiv 2020.09.24.310490; doi: https://doi.org/10.1101/2020.09.24.310490
- Peer reviewed and published scientific report.
Brunaugh, Ashlee D., Hyojong Seo, Zachary Warnken, Li Ding, Sang Heui Seo, and Hugh D. C. Smyth. 2021. “Development and Evaluation of Inhalable Composite Niclosamide-Lysozyme Particles: A Broad-Spectrum, Patient-Adaptable Treatment for Coronavirus Infections and Sequalae.” Edited by Nitesh Kumar Kunda. PLOS ONE 16 (2): e0246803. https://doi.org/10.1371/journal.pone.0246803. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0246803.