Researchers in the United States and Japan have conducted a study showing a common mutation in the spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) - the agent that causes coronavirus disease 2019 (COVD-19) - enhances the infectivity, replication, and early transmission of the virus.
The team’s study of SARS-CoV-2 engineered to harbor the D614G mutation found that this strain was replicated more efficiently in primary human proximal airway epithelial cells than the wildtype virus did.
In a hamster model of infection, the D614G strain also showed much faster respiratory droplet transmissibility than the wildtype virus shortly following infection.
Ralph Baric (the University of North Carolina at Chapel Hill) and colleagues from the University of Wisconsin, University of Tokyo, and the National Institute of Infectious Diseases, Tokyo, say the findings support the need to periodically review SARS-CoV-2 contemporary isolates and identify any new variants with increased transmissibility and pathogenesis that may have emerged.
A pre-print version of the paper is available on the server bioRxiv*, while the article undergoes peer review.
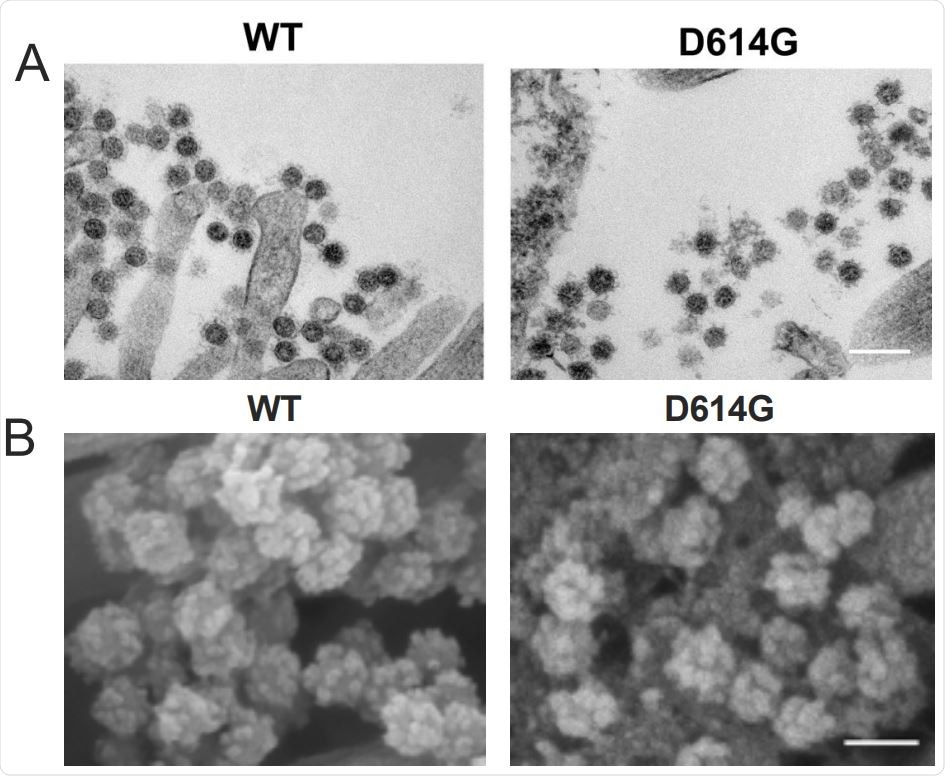
D614G substitution does not alter SARS-CoV-2 virion morphology and S protein cleavage pattern but change viral sensitivity to neutralizing antibodies. A. Transmission electron microscopy image of WT and D614G virions on airway epithelial cell surface, scale bar: 200 nm. B. Scanning electron microscopy images of WT and D614G virions on airway epithelial cell surface, scale bar: 100 nm.
The importance of the spike protein
Since the first cases of SARS-CoV-2 infection were identified in Wuhan, China, late last year, the virus has continued to sweep the globe and has now infected more than 33.8 million people and caused more than one million deaths.
Although most people who become infected only develop mild or asymptomatic disease, some develop severe health complications such as cardiac problems, coagulopathy, stroke, or acute respiratory distress syndrome.
To gain entry to host cells, SARS-CoV-2 uses a surface structure called the spike glycoprotein to bind the human cellular receptor angiotensin-converting enzyme 2 (ACE2).
This spike protein has, therefore, become a central focus of interest in studies aiming to develop vaccines and therapies.
During its pandemic spread in naïve populations, a virus may select for mutations that change its virulence, pathogenesis, or transmissibility.
Studies have recently identified the D614G substitution in the spike glycoprotein as the most prevalent strain of SARS-CoV-2 circulating globally.
However, the effects of this variant on the function, pathogenesis, and transmissibility of SARS-CoV-2 remain unclear.
What did the researchers do?
To investigate the function of the D614G substitution in SARS-CoV-2 replication and transmissibility, the researchers engineered variants containing the D614G mutation in the spike protein, as well as a second variant containing the gene for the bioluminescent reporter nanoLuciferease (nLuc).
The team compared the growth of wildtype SARS-CoV-2 and the D614G variant in primary human nasal epithelia (HNE), large (proximal) airway epithelia (LAE), and distal lung small airway epithelia (SAE).
The D614G-infected HNE and LAE cultures, but not the SAE cultures, exhibited significantly higher viral titers than the wildtype-infected cultures.
Competitive co-infection assays performed in LAE cultures simultaneously infected with both viruses showed that the D614G variant became dominant in the cultures, whether the wildtype virus was originally present at a 1:1 or 10:1 ratio over the D614G mutant.
“These data suggest the D614G substitution enhances SARS-CoV-2 replication fitness in the primary epithelial cells, with a marked advantage in the upper respiratory tract epithelial cells in nasal and large (proximal) airway epithelia,” say Baric and colleagues.
Next, the team performed scanning and transmission electron microscopy to visualize virions present on the surface of primary human airway cell cultures. No significant differences in virion morphology or the number of spike proteins were observed between the two viruses.
Further analysis revealed more differences between the viruses
The researchers used the nLuc-expressing recombinant SARS-CoV-2 encoding either wildtype or D614G spike to measure antibody neutralization activity in serum samples taken from mice vaccinated with D614 (wildtype) spike.
This revealed that the samples half-maximal inhibitory dilution values against the D614G virus were between 0.8 and 5.1 times higher than against the wildtype virus, indicating that the D614G variant makes SARS-CoV-2 more sensitive to neutralizing antibodies.
Evaluating respiratory droplet transmissibility
To evaluate the role of the D614G variant in SARS-CoV-2 respiratory droplet transmissibility, the researchers set up eight pairs of hamsters, each comprising a naïve hamster alongside an infected animal 1 day following infection.
Both the wildtype and D614G viruses were efficiently transmitted to naïve hamsters. At 4 and 6 days following infection, the infected hamsters and the exposed hamsters exhibited similar viral titers, regardless of which virus they had been infected with.
However, five of eight hamsters exposed to the D614G-infected group showed infection and had detectable viral shedding on day 2, while those exposed to the wildtype-infected group showed no infection or viral shedding. This suggests that the D614G variant is transmitted much more quickly between hamsters via droplets and aerosols than the wild type virus is.
“Our study demonstrated the SARS-CoV2 D614G substitution enhances infectivity, replication fitness, and early transmission,” conclude the researchers.
“Our data support the critical need to periodically review SARS-CoV-2 contemporary isolates across the globe and identify the emergence of new variants with increased transmission and pathogenesis and/or altered antigenicity, especially as levels of human herd immunity and interventions alter the selective forces that operate on the genome,” advises the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Baric R, et al. SARS-CoV-2 D614G Variant Exhibits Enhanced Replication ex vivo and Earlier Transmission in vivo. bioRxiv, 2020. doi: https://doi.org/10.1101/2020.09.28.317685
- Peer reviewed and published scientific report.
Hou, Yixuan J., Shiho Chiba, Peter Halfmann, Camille Ehre, Makoto Kuroda, Kenneth H. Dinnon, Sarah R. Leist, et al. 2020. “SARS-CoV-2 D614G Variant Exhibits Efficient Replication Ex Vivo and Transmission in Vivo.” Science 370 (6523): eabe8499. https://doi.org/10.1126/science.abe8499. https://www.science.org/doi/10.1126/science.abe8499.