Researchers in the United States and Canada have discovered a monoclonal antibody that potently neutralizes severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the agent responsible for the current coronavirus disease 2019 (COVID-19) pandemic.
The team used a high-throughput microfluidic screening technique that allowed rapid identification of the antibody, called LY-CoV555.
This antibody targets explicitly the receptor-binding domain (RBD) of the SARS-CoV-2 viral spike protein, the main structure the virus uses to infect host cells.
Biochemical, structural, and functional characterization analyses showed that LY-CoV555 exhibited high-affinity binding to the RBD and stopped SARS-CoV-2 binding to the human host cell receptor angiotensin-converting enzyme 2 (ACE2).
In a rhesus macaque model, the agent reduced viral replication in the upper and lower airways, at doses as low as 2.5 mg/kg, indicating the potential for reduced viral shedding and transmission following treatment.
“These data supported the progression of LY-CoV555 into clinical evaluation,” say Bryan Jones (Eli Lilly and Company, San Diego) and colleagues.
The researchers also say their study demonstrates that high-throughput screening can lead to the identification of a potent antiviral antibody that protects against SARS-CoV-2 infection.
A pre-print version of the paper is available on the server bioRxiv*, while the article undergoes peer review.
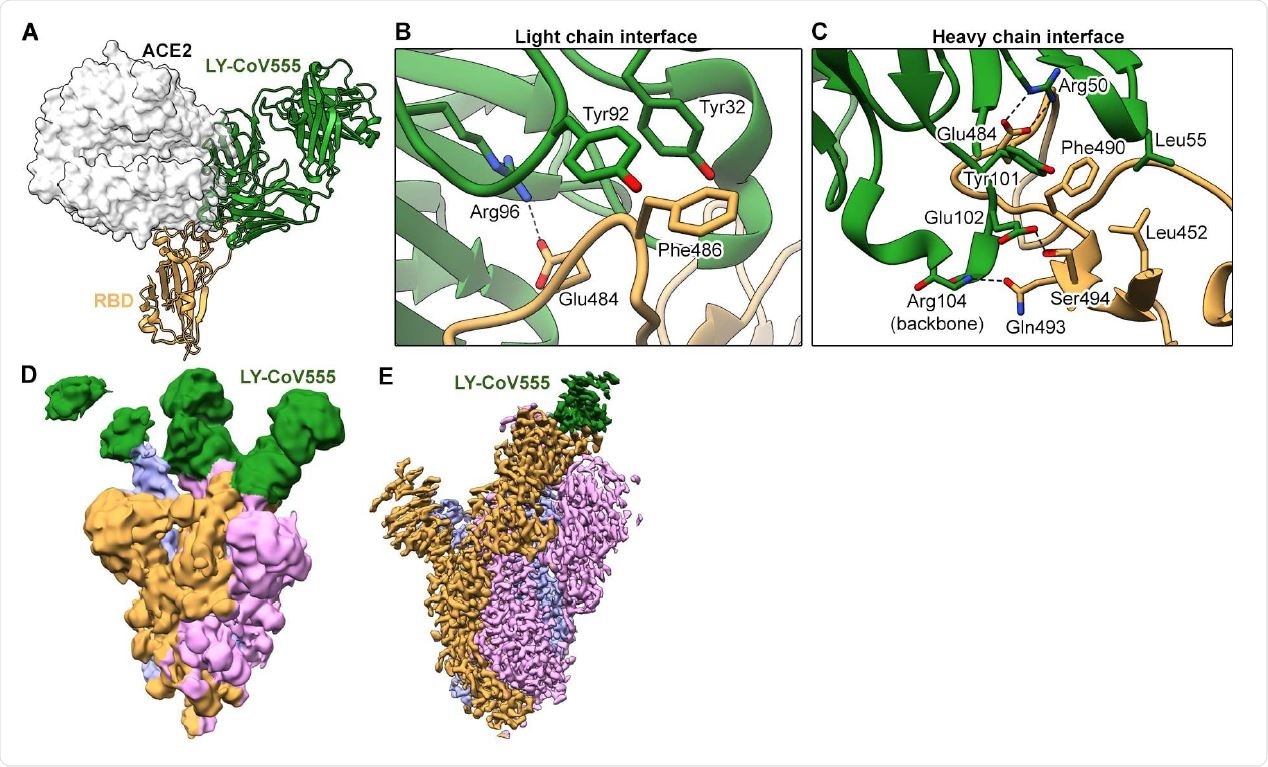
LY-CoV555 blocks ACE2 and binds to the spike protein RBD in the up and down conformations. (A) Crystal structure of the RBD-LY-CoV555 complex superimposed with the ACE2 receptor from a structure of the RBD-ACE2 complex (PDB ID: 6M0J) (26). Zoomed-in view of key atomic interactions at the interface of the LY-CoV555 light chain (B) and heavy chain (C) with the spike RBD. (D) Cryo-EM structure of the LY-CoV555-spike complex low-pass filtered to 8Å resolution and shown at low threshold in order to visualize all 3 Fabs (shown in green). (E) High-resolution cryo-EM map of the LY-CoV555-spike complex. Cryo-EM = cryo-electron microscopy; RBD = receptor-binding domain.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The race to develop therapies
As SARS-CoV-2 continues to rapidly spread across the globe, posing a significant threat to public health and the economy, researchers are racing to develop therapeutic agents that could be of clinical benefit until a vaccine becomes available.
Neutralizing antibodies against SARS-CoV-2 are a central focus of research efforts, which have mainly concentrated on targeting the surface spike protein that enables the virus to gain access to host cells.
Viral entry is initiated by upward movement of the RBD at the apex of the spike protein, which enables binding to ACE2. The S2 subunit of spike then undergoes conformational rearrangement to allow fusion to the host cell membrane and the delivery of viral genetic material into the cell.
“Given the critical nature of the RBD interaction with ACE2 for viral entry, antibodies that bind the RBD and interfere with ACE2 binding can have potent neutralizing activity,” write Jones and colleagues.
High-throughput microfluidic screening quickly identified neutralizing antibodies
Now, the team has described a high-throughput microfluidic screening of antigen-specific B-cells that enabled the rapid identification of anti-spike neutralizing antibodies.
This quickly led to the discovery, isolation, and characterization of LY-CoV555, a potent RBD-specific neutralizing antibody derived from a convalescent COVID-19 patient.
Biochemical, structural, and functional characterization revealed that LY-CoV555 bound with high affinity to the spike RBD and exhibited potent ACE2 blocking properties and virus-neutralizing activity.
Testing the treatment in rhesus macaques
To assess antibody’s ability to protect against SARS-CoV-2 infection, rhesus macaques were intravenously administered 1, 2.5, 15 or 50 mg/kg doses of LY-CoV555 or 50 mg/kg of a control antibody 24 hours prior to virus challenge.
The team reports that this prophylactic treatment protected against infection in the lower respiratory tract.
In bronchoalveolar lavage (BAL) samples taken from the animals, viral replication and load were significantly reduced, compared with controls, at days 1, 3, and 6, following viral challenge.
Among the animals treated with LY-CoV555, viral replication in BAL was undetectable by day 3 at all doses administered.
LY-CoV555 also protected against infection in the upper respiratory tract. Viral load was significantly reduced in the throat at day 1 and in the nose at days 3 and 6 across all doses given.
Most importantly, viral replication was undetectable in the nose by day 3 at all dosage levels.
“Modest doses of LY-CoV555 could provide substantial clinical efficacy”
“Prophylaxis doses as low as 2.5 mg/kg reduced viral replication in the upper and lower respiratory tract,” writes the team. “We hypothesize that modest doses of LY-CoV555 could provide substantial clinical efficacy.”
The researchers say the findings also demonstrate that high-throughput screening can lead to the identification of an effective antiviral antibody that protects against SARS-CoV-2 infection.
“Overall, the identification and characterization of LY-CoV555 points to the feasibility of strategies to rapidly identify neutralizing human mAbs [monoclonal antibodies] as part of an initial response to an evolving pandemic that can complement population-scale vaccination, provide immediate passive immunity, and provide protection for vulnerable populations,” concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Jones B, et al. LY-CoV555, a rapidly isolated potent neutralizing antibody, provides protection in a non-human primate model of SARS-CoV-2 infection. bioRxiv, 2020. doi: https://doi.org/10.1101/2020.09.30.318972
- Peer reviewed and published scientific report.
Jones, Bryan E., Patricia L. Brown-Augsburger, Kizzmekia S. Corbett, Kathryn Westendorf, Julian Davies, Thomas P. Cujec, Christopher M. Wiethoff, et al. 2021. “The Neutralizing Antibody, LY-CoV555, Protects against SARS-CoV-2 Infection in Nonhuman Primates.” Science Translational Medicine 13 (593): eabf1906. https://doi.org/10.1126/scitranslmed.abf1906. https://www.science.org/doi/10.1126/scitranslmed.abf1906.