As COVID-19 continues to haunt the world, vaccine development efforts are moving ahead in many different countries. It is hoped that effective vaccines will help break the chain of transmission and stranglehold of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on global health and financial activity.
Previous Results from the Vaccine
Most of the vaccines under development focus on the viral spike (S) protein, which is considered to be necessary for the entry of the virus into the host cell to achieve infection. The authors of the current study have shown the potential for an inactivated SARS-CoV-2 vaccine where the S, nucleoprotein (N) and other viral antigens are all exposed to the immune cells.
A trial in nonhuman primates showed that this vaccine was capable of protecting them against the virus when faced with a challenge dose. This led to a phase I clinical trial. The current paper describes the safety and immunogenicity of the vaccine in human beings in this phase I trial.
The study included 192 adults between the age of 18 and 59 years, randomized to the vaccine, or a placebo. From May 2020 through August 2020, all participants who received two inoculations of the vaccine or placebo were monitored for any clinical manifestations and were required to provide blood samples 3 (0, 14 schedule) or 2 (0, 28 schedule) times after inoculation. The withdrawal rate was 0.5%: 1 participant in the low-dose group who was assigned to the 0, 28 schedule did not receive the second dose. The 191 participants were divided as follows: 24 in each of the three different dose groups and the control group assigned to the 0, 14 schedule and 23, 24, 24 and 24 in the low-dose, medium-dose, high-dose and control groups assigned to the 0, 28 schedule.
Safety is a Number 1 concern with any new vaccine or treatment. The current authors explored the possibility that this vaccine would cause abnormal activation of the immune system. They looked at solicited and unsolicited adverse events for up to 7 days from each inoculation and 28 days from immunization, respectively. No serious adverse reactions were observed in either group.
Up to 4 patients in each dosage group of immunized patients had solicited systemic adverse reactions, both immunized and placebo recipients. Unsolicited reactions were found in up to up to ~8% and ~13% of immunized patients, at the 0, 14 day group, and 0, 28 day group, respectively, vs. zero and ~4% in the respective placebo groups.
They also examined the variations in the cytokine levels and T cell counts in the blood of half the inoculated patients, whether they had received a placebo or not. There was no evidence of a cytokine storm. Moreover, antibody-dependent enhancement (ADE) of disease was not apparent in a dilution experiment using sera from immunized individuals, across a serial range of dilutions.
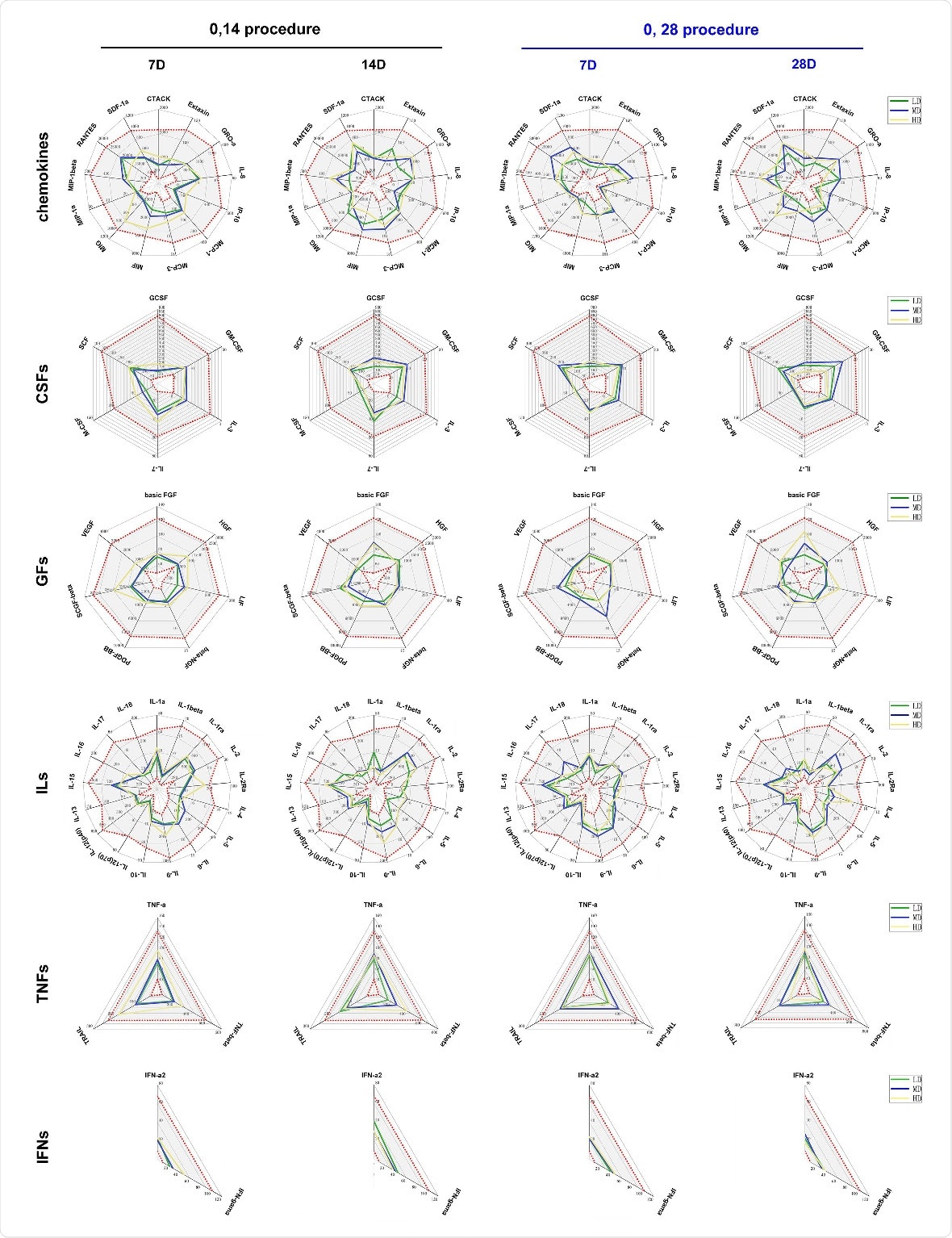
Variations in 48 cytokines in the serum of immunized individuals and the observation of ADE. Levels of 48 cytokines were monitored in the serum of the subjects who received the vaccine and placebo who were assigned to the 0, 14 schedule (black) or the 0, 28 schedule (blue). Cytokines were Chemokines, interleukins (ILs), growth factors (GFs), colony stimulating factors (CSFs), tumor necrosis factors (TNFs), interferon (IFNs). The levels of 48 cytokines (pg/mL) in the serum of subjects before receiving vaccine and placebo are shown as gray intervals between red spots in each figure. Control (Con, 0 EU), low dose (LD, 50 EU), middle dose (MD, 100 EU) and high dose (HD, 150 EU).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Synchronous and Increasing Immune Response
In both groups, neutralizing antibodies were produced in 55%, 100%, and 88% in low-, medium- and high-dose groups at day 7 from the second dose. At day 14, the seroconversion rates were 92%, 100% and 96%, respectively.
The antibody titer apparently declined between day 14 and 28 in the 0, 14 day group but not in the 0, 28 day group. The immunized patients uniformly showed similar increases in antibodies against the viral S and N antigens and the whole virion and T cell responses, showing that the vaccine induces a synchronous and increasing humoral and cellular immune response to the virus.
The vaccine elicited IgG1 antibodies against all three antigens, with an overall neutralizing antibody seroconversion of ~91% but 100% ELISA- detectable antibodies. Notably, the sera from the immune patients neutralized infection by all the viral strains circulating in North America carrying the D614G mutation, which confers increased infectivity.
Immunogenicity of the vaccine
The researchers also found that the vaccine upregulates a multitude of genes related to the immune response, indicating that it activates both innate and adaptive arms of the immune system. Simultaneously, it does not appear to stimulate pro-inflammatory signaling pathways such as those mediated by IL-5 and IFN-γ. There was an increase in B and T cell activation by 40% and 25% beginning around day 7 from the booster dose. The vaccine also activated genes related to the function of innate immune cells, adding to the spectrum of the immune response.
Implications
The study concludes that there is no evidence of severe adverse reactions, cytokine storm, or ADE following immunization with this vaccine. Secondly, it appears capable of inducing a neutralizing response in proportion to the dose and timing of vaccination, with 100% seroconversion of the ELISA anti-S antibody following two doses.
Finally, the neutralizing antibody is capable of protecting against infection by diverse pandemic strains with diverse mutations. Both humoral and cellular antibody responses were observed in the vaccinated individuals, with specific T cell activation in response to any of the three antigens.
The authors conclude, “All the data obtained in this trial support the safety and immunogenicity of this inactivated vaccine and are encouraging with regard to further studies of its efficacy in the future.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Pu, J. et al. (2020). An in-depth investigation of the safety and immunogenicity of an inactivated SARS-CoV-2 vaccine. medRxiv preprint. doi: https://doi.org/10.1101/2020.09.27.20189548. https://www.medrxiv.org/content/10.1101/2020.09.27.20189548v1
- Peer reviewed and published scientific report.
Pu, Jing, Qin Yu, Zhifang Yin, Ying Zhang, Xueqi Li, Qiongzhou Yin, Hongbo Chen, et al. 2021. “The Safety and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine in Chinese Adults Aged 18–59 Years: A Phase I Randomized, Double-Blinded, Controlled Trial.” Vaccine 39 (20): 2746–54. https://doi.org/10.1016/j.vaccine.2021.04.006. https://www.sciencedirect.com/science/article/pii/S0264410X2100431X.