Researchers in the United States and Sweden have created personalized models of fetal brain microglia to investigate the effects of maternal infection on the developing brain, including infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent that causes coronavirus disease 2019 (COVID-19).
Microglia are macrophages found in the brain tissue that plays a key role in neurodevelopment, but inappropriate fetal microglial priming or “trained immunity” as a result of exposure to in utero immune activation may pose a risk to the developing fetal brain.
Now, researchers from Massachusetts General Hospital and the Karolinska Institute in Stockholm have shown that umbilical cord blood-derived mononuclear cells (CB-MNCs) can provide a non-invasive, individualized model of fetal brain microglial priming.
The researchers demonstrate the application of this approach by generating microglia from cells that had been exposed and unexposed to maternal SARS-CoV-2 infection.
“These models should facilitate novel insights into fetal brain development in the setting of maternal exposures, including but not limited to SARS-CoV-2 infection,” say Andrea Edlow (Massachusetts General Hospital) and colleagues.
A pre-print version of the paper is available in the server bioRxiv*, while the article undergoes peer review.
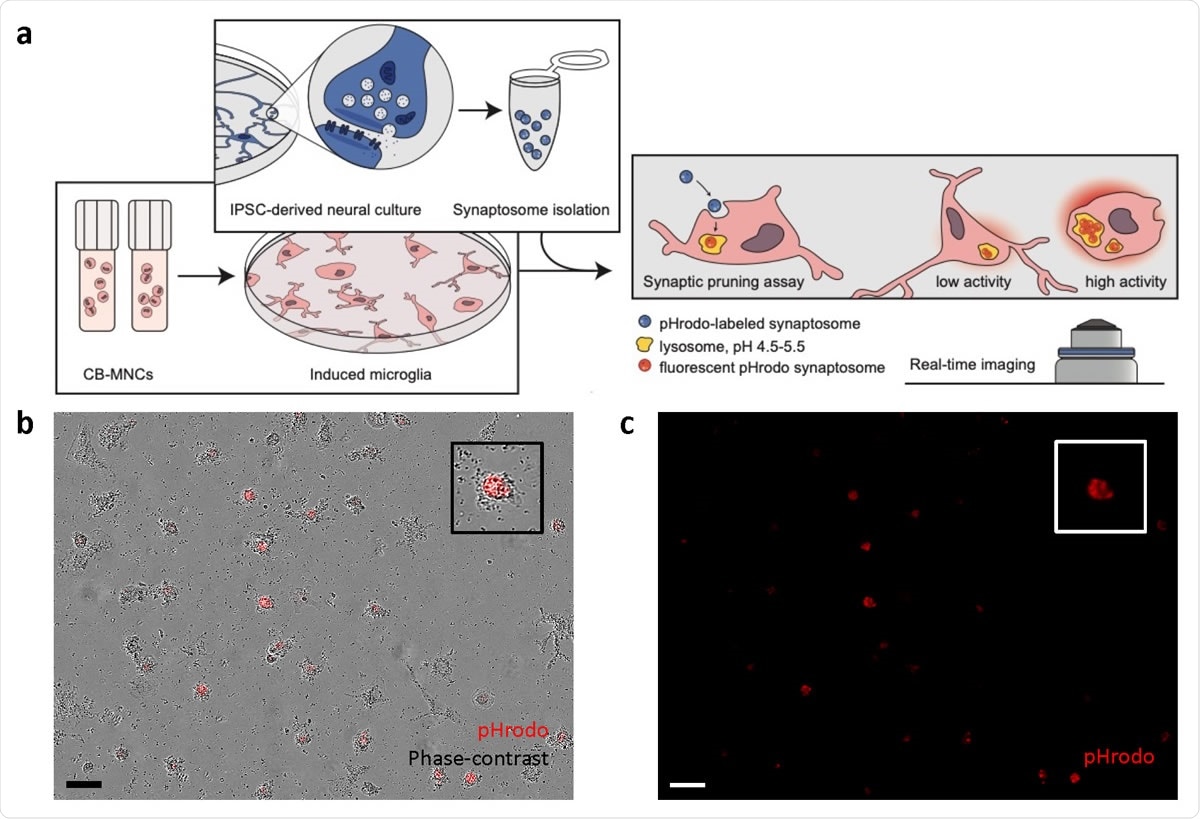
Synaptosome engulfment functional characterization of CB-iMGs in an in-vitro model of synaptic pruning. (a) Overall schematic of pHrodo-labelled quantitative synaptosome phagocytosis assay by CB-iMGs. (b) Representative live real-time images in phase-contrast/red fluorescence overlay mode showing cellular uptake and (c) red fluorescence channel alone of pHrodo (red)-labeled synaptosomes uptake after 5 h. Scale bar: 60 μm (Boxes show magnified view of engulfing CB-iMG).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Various different exposures can trigger in utero immune activation
In utero immune activation can be triggered by exposures ranging from metabolic conditions to stress and infection, with potential adverse impacts on the developing fetus during pregnancy.
In particular, some studies have suggested that maternal viral and bacterial infections may be associated with adverse neurodevelopmental outcomes among offspring.
Although the processes underlying these adverse outcomes remain unclear, microglial priming towards a pro-inflammatory phenotype and subsequent alteration of synaptic pruning has been proposed as a potential mechanism.
“The central role of mononuclear cells, including macrophages, in COVID-19 pathogenesis, suggests that the potential risk to exposed fetal microglia requires investigation,” say Edlow and colleagues.
However, there are currently no biomarkers or models for in utero microglial priming that might help to identify the infants most vulnerable to neurodevelopmental problems because the microglia are inaccessible in the fetus and following birth.
Previously, the researchers created and validated personalized adult models of microglia-mediated pruning by reprogramming induced microglial cells from peripheral blood mononuclear cells (PBMCs) and assaying them with isolated synapses (synaptosomes) derived from neural cultures differentiated from induced pluripotent stem cells.
Adapting this previous method for application to CB-MNCs
Now, the team has tested whether this method can be adapted and applied to CB-MNCs derived from pregnancies exposed and unexposed to SARS-CoV-2, to develop models of fetal brain microglia that are unique to each neonate.
The researchers showed that the CB-MNCs could be reprogrammed to create neonatal patient-specific models of microglia-mediated synaptic pruning.
The team reports that microglia-like cells derived from CB-MNCs that had been both exposed and unexposed to SAR-CoV-2 expressed canonical microglial markers and had varying morphologies with different degrees of ramification.
This potentially reflects a range of different activation states that can be perturbed in experimental systems, say Edlow and colleagues.
Importantly, the team saw that induced microglia phagocytosed synaptosomes, suggesting that this could also occur in the developing brain.
What are the implications of the study?
“This work suggests the potential for umbilical blood mononuclear cells to serve as a non-invasive, personalized biomarker of fetal brain microglial priming,” write the researchers.
The team says that extending their work with adult PBMCs to cord blood-derived microglial models will allow for rapid, scalable models that can be used to investigate risk of neurodevelopmental abnormalities and provide non-invasive, individualized assays of the impact SARS-CoV-2 has on fetal brain microglial priming and synaptic pruning function.
Furthermore, the ability to detect the priming of fetal brain microglia towards a pro-inflammatory phenotype applies to various maternal infections beyond SARS-CoV-2 and other types of maternal exposures that have been suggested to affect the development of fetal microglia.
The approach could lead to the investigation of targeted therapeutic strategies
“To our knowledge, microglia have not previously been modeled from umbilical cord blood or used to predict neurodevelopmental vulnerability at a time when there is a window for intervention,” say Edlow and colleagues
“Beyond characterizing any consequent abnormalities, the scalability of this approach may enable investigation of targeted therapeutic strategies to rescue such dysfunction,” concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Edlow A, et al. Umbilical cord blood derived microglia-like cells to model COVID-19 exposure. bioRxiv, 2020. doi: https://doi.org/10.1101/2020.10.07.329748
- Peer reviewed and published scientific report.
Sheridan, Steven D., Jessica M. Thanos, Rose M. De Guzman, Liam T. McCrea, Joy E. Horng, Ting Fu, Carl M. Sellgren, Roy H. Perlis, and Andrea G. Edlow. 2021. “Umbilical Cord Blood-Derived Microglia-like Cells to Model COVID-19 Exposure.” Translational Psychiatry 11 (1): 1–9. https://doi.org/10.1038/s41398-021-01287-w. https://www.nature.com/articles/s41398-021-01287-w.