COVID-19 has ravaged the population worldwide, mostly causing respiratory symptoms. A sizable percentage of patients have developed acute respiratory distress syndrome (ARDS) and multi-organ failure, and over a million have died. Older and sicker individuals are disproportionately prone to severe or critical disease.
In many of these patients, the virus has been found in the bloodstream and in organs other than the lung. In one series of autopsies carried out on a large group of patients who had succumbed
to the infection, the investigators discovered the presence of pulmonary artery embolism or deep vein thrombosis in ~40% of cases. This shows that the virus produces abnormalities in coagulation. In five younger patients, stroke due to the involvement of large vessels, including the carotid artery in at least one case, was shown to have occurred.
Other instances of vascular abnormalities associated with COVID-19 have been shown. These include brain-related symptoms. The underlying pathology may be inflammation of the endothelial lining of the blood vessels.
High Viral Loads in Carotid Samples
In the current study, the researchers used real-time PCR to measure viral RNA titers in the typical carotid artery samples from 32 patients, with matched lung and throat samples. In all cases, the viral loads in the lung were high, indicating the presence of viral pneumonia. Additionally, in over 80% of the samples, viral RNA was found in the carotid arteries, with the viral loads being similarly high in this tissue.
Microscopic examination of carotid artery tissue showed the vessels to show the expected features for the patient's age, but with moderate inflammation. No signs of endothelial inflammation or vasculitis were observed.
The researchers then tried to isolate infectious viral particles from the carotid artery samples and another 10 matching samples of tissue from 7 patients. In five of these patients, viremia had been confirmed before death. The virus was successfully isolated from 75% of the carotid artery and saphenous vein samples and from the lung, throat, and jejunum.
Replicating Infection Established
The viral RNA from all these sites was sequenced and found to be identical in the lung and carotid artery samples from the same patients. However, there were single nucleotide variations in the strains sequenced from different patients. All recovered viral RNA sequences belong to the most commonly circulating European strains.
The researchers concluded that infectious virus could be isolated from the organs of non-surviving patients only if viremia occurs before death, and the rate of isolation closely corresponds with the viral load in each organ. However, they could not retrieve the virus from other tissues such as the liver or the brain.
The presence of replicating virus in both lung and five of the seven carotid arteries was confirmed by finding plentiful subgenomic SARS-CoV-2 RNAs. The remaining two artery samples showed low RNA quality and could not be sequenced. The replication levels were as high as or higher than those observed in the lung samples.
The authors conclude, "Together these data indicate that SARS-CoV-2 infects and replicates in carotid arteries."
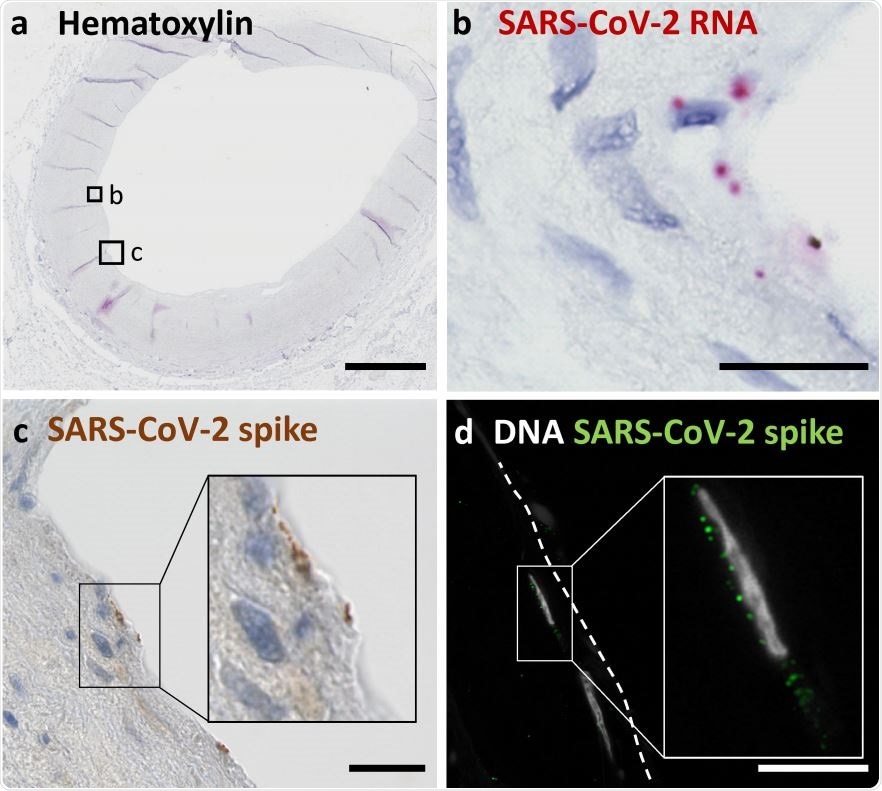
(a) Overview shows a cross section of an A. carotis counterstained with Hematoxylin and subjected to in situ SARS-CoV-2 RNA hybridization. (b) Close up of boxed region B in (a). (c) Close up of boxed region C, which depicts immunohistochemical staining of SARS-CoV-2 spike protein in a section consecutive to (a). (d) Immunofluorescence staining of nuclei and spike protein in endothelial cells seen in a section consecutive to the section shown in (a). Scale bars represent 300 μm (a) and 20 μm (b, c, d).

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Endothelial Infection Confirmed
Examining the carotid artery structure in detail, they used SARS-CoV-2 plus-strand RNA in situ hybridization, and immunological methods like immunofluorescence and immunohistochemistry directed against the viral spike antigen, to detect the exact cells in the carotid artery tissue that harbored the actively replicating virus. This showed that the viral RNA was in the endothelial layer.
Transcriptomics in arterial tissue from two of the patients, vs. two control patients without COVID-19, showed reduced expression of type I and type II interferon pathways in the SARS-CoV-2 infected arteries. This agrees with recent reports showing that innate immunity and interferon-related signaling pathways are downregulated in cells infected by this virus and animal models.
Immunosuppressive Responses
The researchers found that genes which should be strongly expressed in case of endothelial inflammation and angiogenesis, such as VCAM and NFκB, and TIE1 and EGR1, respectively, were instead markedly downregulated in the infected arteries.
This indicates, they say, "that SARS-CoV-2 infection induces a strong anti-inflammatory and anti-proliferative state in blood vessels."
This finding is also in agreement with recent studies that show the virus to have a significant modulatory effect on immunity. Further studies will be needed to confirm this effect, however, given that these patients had acute myeloid leukemia and myelofibrosis, respectively, which may have contributed to the immunosuppression.
Implications
The investigators postulate that once viremia occurs, the virus infects the vascular endothelium and establishes replication.
This leads to a downregulation of normal vascular responses, and the further spread of the virus into multiple organs. This could explain the occurrence of the pediatric condition called Kawasaki disease in which medium-caliber arteries are inflamed, in children with COVID-19. More research will undoubtedly shed light on the role played by carotid artery and other vascular infection by the virus in the pathogenesis, clinical phenotype and organ involvement in COVID-19.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.