The current COVID-19 pandemic is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a betacoronavirus that causes severe acute respiratory distress in a significant proportion of infected patients. A recent study published on the preprint server medRxiv* in October 2020 reports a new diagnostic test for SARS-CoV-2 based on the detection of antibodies.
At present, testing for SARS-CoV-2 is carried out by the detection of viral RNA using Reverse Transcribed Real-Time PCR (RT-PCR) or by radiological methods such as computed tomography (CT) imaging of the chest or rapid antigen/antibody testing.
Early immunity in viral infections is via the development of IgM antibodies, followed by adaptive immunity in the form of IgG antibodies with high specificity and affinity for the viral antigens. These are capable of neutralizing the virus, preventing its entry into and infection of the host cell. Other memory B cells can provide durable immunological memory and long-term immunity.
Serological Studies
The current study focuses on the testing of specific antibodies to the virus in order to develop a diagnostic test that is rapid, simple, and sensitive. The development of antibodies to the virus indicates prior exposure to the virus and an immune response.
Earlier studies show that seroprevalence varies widely, with one study reporting IgM and IgG antibodies in over ~83% and ~65% of patients, for a total prevalence of ~93%. The pattern of progression appears to be first the appearance of total antibody, then of IgM, and finally IgG. The most significant proportion of antibodies are against the viral nucleocapsid (N) protein, which is the most abundant. Tests that detect are, therefore, the most sensitive among antibody tests.
On the other hand, the spike protein binds to the host cell receptor through the receptor-binding domain, and this would therefore elicit the most specific antibodies, which are also considered to be neutralizing. Prior research, therefore, recommends the use of either or both the spike and N proteins to achieve the greatest sensitivity.
The current study was aimed at developing a new IgM/IgG rapid antibody test against this virus, based on lateral flow assay (LFA). It was evaluated by the gold standard test, RT PCR, and Electro-chemiluminescence immunoassay (ECLIA) to measure the total antibody titer.
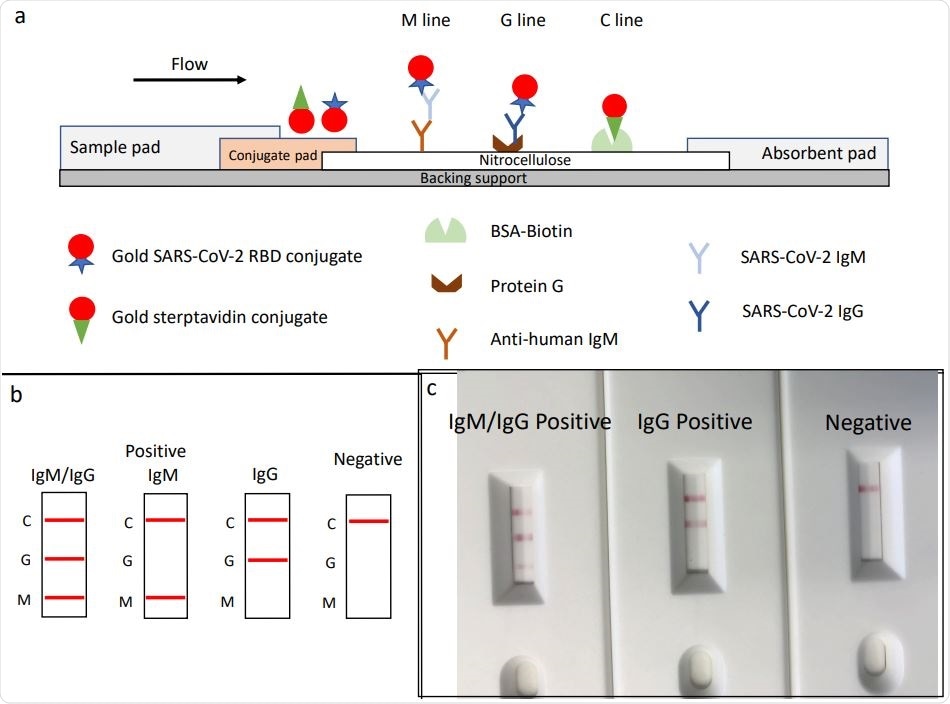
Schematic illustration of rapid SARS‐CoV‐2 IgM‐IgG combined antibody test and example of typical results. a, Schematic diagram of the detection device; b, illustration of different testing results; c, example of typical results obtained with the RDT.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Developing the Rapid Test
The researcher Percevent J Ducrest. CEO & co-founder of GaDia SA developed a lateral flow immunoassay that could rapidly identify the presence of IgM and IgG against the virus in human samples when carried out in a setting with high COVID-19 prevalence. Using a strip that shows a red line if antibodies are present in either the M or the G region, Ducrest was able to differentially pick up either IgM or IgG.
The antibody in the sample binds to the RBD antigen coated on gold nanoparticles. This complex moves through the strip, with the antibodies binding to immovable substances in the strip, which capture the IgM or IgG antibodies respectively to form the M or G lines.
In the absence of any antibody to the virus, the colloidal gold moves up and binds at the control line at the end of the strip, caught to BSA-biotin. This forms a red line and must be seen to ensure a valid test has occurred, irrespective of the result.
Baseline characteristics
The baseline characteristics of the patients whose samples were tested in the current study show that their median age was 50 years, vs. 33 for the controls. The male: female ratio was similar for both patients and controls. The median period to sampling from symptom onset is 21 days, and all the RT PCR testing was carried out in nasal swabs. The median cycle time value was ~26.
The researcher carried out ECLIA on 11/35 RT PCR positive samples. Ducrest found that the IgG/IgM rapid diagnostic test (RDT) agreed with the PCR in ~90% of cases. In 7 cases, the results were positive for IgM only, showing the specificity of 93% for IgG/IgM, but 100% for IgG only.
Sensitivity of IgG/IgM RDT vs RT PCR
The IgG/IgM RDT showed 100% sensitivity for either IgG, IgM, or both. A positive result was likely to be a true infection in 83%, while a negative result indicated the absence of infection in all cases when matched with the RT PCR results. In two cases, patients were still antibody positive at day 170 and 180 from symptom onset.
Sensitivity of IgG/IgM RDT vs. ECLIA
Ducrest found that compared to ECLIA, the sensitivity for either or both antibodies was 100%, but only 61% of positive values were truly infected. The negative results by ECLIA were all negative by RDT also.
Ducrest concludes that evaluated in a cohort of cases matched with controls, the IgG/IgM RDT on plasma samples is as accurate as a diagnostic tool as RT PCR or ECLIA, with 100% sensitivity and negative predictive value, and 93% specificity. Thus, this test is capable of diagnosing all infected individuals and correctly ruling out infection in 93% of uninfected individuals.
When compared to ECLIA, a positive result correctly predicts infection in 61% of cases, while in comparison with RT PCR, the positive predictive value is 83%. This RDT failed to yield even one false negative, indicating its 100% sensitivity even with borderline samples. Its performance at a cut-off similar to the ECLIA manufacturer Roche is excellent, even though the latter targets the antibodies against the full-length N protein, and the RDT identifies only anti-RBD antibodies.
Implications and Future Directions
For serological diagnosis, testing should be carried out only 10-15 days from the earliest symptom and not in the early phase of infection. Notably, these tests used plasma, and further research will be needed to understand how the test works in a point-of-care setting with capillary blood.
These tests showed IgG and IgM seropositivity even 180 days from the onset of symptoms, and this is the first time an RDT has done so at this time point, say the authors. This shows the constant and sufficiently high titer of these antibodies over the period of 15-180 days post symptoms, allowing detection with this particular RDT.
Further research is necessary to measure the titer of IgM and IgG at this point in order to validate these findings. Interestingly, the RDT provided only determinate results.
This preliminary study only provided a proof of concept and was tested on a population with a high prevalence of positive cases, accounting for the high PPV. In a low-prevalence population, this may not be so. However, when the population is tested 10 days from the onset of symptoms
Ducrest comments, “Such performances indicate that this RDT could be fit for purpose in clinical settings where a high prevalence of COVID-19 prevails, especially in situations where ECLIA are not available, or cannot be reliably used. Diagnostic performances in low prevalence populations still needs to be determined, and larger populations need to be tested.”

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.