COVID-19 is known to be associated with multiple changes in gene expression, but many of these are also seen in chronic brain conditions as well as in some abnormal mental conditions. A recent study published on the preprint server bioRxiv* in October 2020 uncovers the molecular basis of this association.
Though severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes predominantly respiratory symptoms, it is becoming clear that many other organ systems can also be involved. Symptoms related to the brain include anosmia, headache, memory loss, cognitive deficits including loss of concentration, and chronic tiredness, especially common in previously hospitalized COVID-19 patients.
Such symptoms could be due to direct neuronal and glial infection by the virus or indirect damage due to the infection and subsequent inflammation. The long-term persistence of such symptoms occurs in both SARS and MERS.
Some studies have detected the presence of SARS-CoV-2 RNA in the olfactory mucosa and the cerebrospinal fluid (CSF). On the other hand, experiments have failed to prove infection in neuronal organoids, and the relevance of these systems has not been established.
Thirdly, the physiological profile of many brain regions significantly affected by COVID-19 remains unclear.
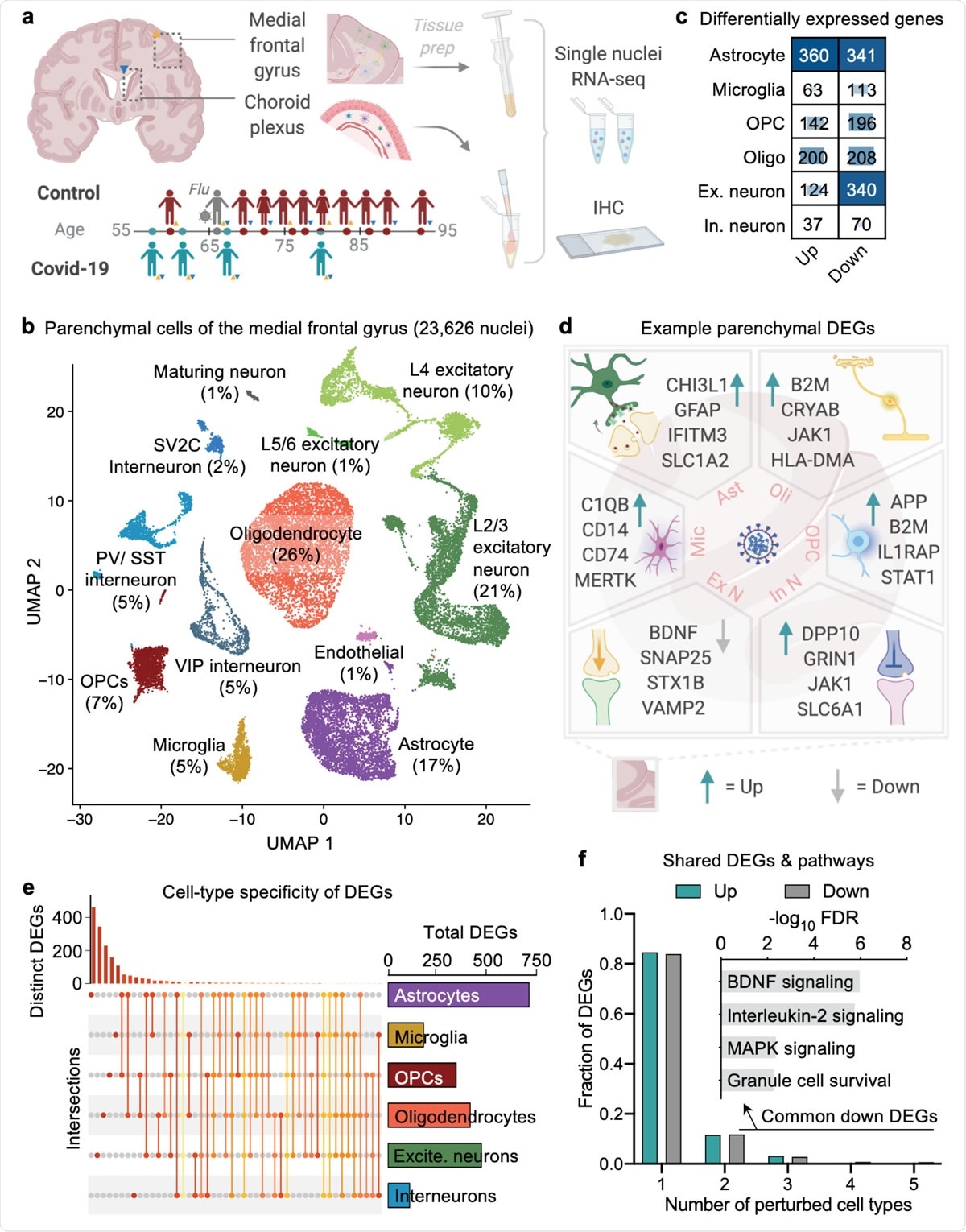
Cell type-specific gene expression changes in the brain of COVID-19 patients. a, Study design. Colored triangles denote brain regions studied for each patient. b, Uniform Manifold Approximation and Projection (UMAP) of 23,626 nuclei captured from the human medial frontal cortex, colored by cell type and labeled with percent of total nuclei. Note, that as in prior reports32,34,35, the ‘Endothelial’ cluster also exhibits vascular mural cell markers. c, Differentially expressed gene (DEG) counts for each cell type (MAST with default thresholds, FDR < 0.05, Log (fold change) > 0.25 (absolute value)). The intensity of the blue color and the size of the squares are proportional to entry values. d, Example differentially expressed genes (DEGs) in COVID-19: excitatory (Ex N) and inhibitory (In N) neurons, astrocytes (Ast), oligodendrocytes (Oli), oligodendrocyte precursor cells (OPC), and microglia (Mic). e, Matrix layout for intersections of DEGs shared across and specific to each cell type. Circles in the matrix indicate sets that are part of the intersection, showing that most DEGs are cell type-specific. f, Fraction of total up- and downregulated genes (y-axis) as a function of the total number of cell types in which the differential expression occurs. Biological pathways associated with genes downregulated in COVID-19 that are common to ≥2 cell types are shown (n = 161 genes, hypergeometric test, FDR correction).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Cell-Specific DEGs in COVID-19
The current study uses single-nucleus transcriptomics to study neurons in the brain's frontal cortex and choroid plexus. The researchers obtained samples from controls and COVID-19 patients between 58 and 91 years of age. Among these patients, four had COVID-19, four did not have COVID-19, and one had influenza.
All patients with lethal COVID-19 and influenza had spent two or more weeks on mechanical ventilators before succumbing to interstitial pneumonia. Two had neurological symptoms during their hospital stay.
The researchers identified eight types of cells by gene marker, including excitatory and inhibitory neurons, astrocytes, oligodendrocytes, oligodendrocyte progenitor cells (OPCs), microglia, maturing neurons, and endothelial cells.
In the COVID-19 samples, they found ~2,2000 unique differentially expressed genes (DEGs) spread over all cell types, most frequently in the astrocytes, excitatory neurons, and oligodendrocytes. Three-quarters of the DEGs in excitatory neurons and two-thirds of interneurons were downregulated, fitting the repression signature found in Alzheimer's disease (AD) but not in other cell types.
Specific responses to COVID-19 were observed, with 84% of the DEGs being confined to only one cell type. The remaining shared DEGs followed distinct pathways, such as the frequent downregulation of BDNF and IL-2, both required for neuronal homeostasis.
The researchers comment, "These results indicate that all major brain parenchymal cell types are affected by COVID-19, and that single-cell-level resolution is critical because changes in gene expression—including directionality—are dependent on cell type."
High SARS-CoV-2 Titers in Barrier Cells Stimulate Inflammatory Signaling
Earlier experiments that showed virus invasion of brain organoids did not have barrier cells. The current study shows the presence of the viral spike glycoprotein in the same frontal and choroid plexus tissue with these DEG profiles. The viral spike signal was localized to the cortical blood vessels' barrier cells, brain coverings, and choroid plexus. However, there was no observable viral infection of the cortical parenchyma, though undetectable infection may be present, of course.
Thus, the brain-blood barrier cells allow viral entry and replication at low viral titers, allowing viral accumulation and resulting DEGs across the cortex. They found that the antiviral gene IFITM3 was expressed at higher levels. Such an elevation is a marker for COVID-19 and occurred in brain vascular cells and a range of endothelial, epithelial, and supporting cells in the stroma.
Brain parenchyma also showed high levels of the alternative viral entry receptor NRP. This is known to be high in inflamed lung tissue in COVID-19, suggesting CNS inflammation in this infection.
They also reported raised levels of IL-1β, CCL2, and CCL4, capable of activating microglia and astrocytes to produce CNS inflammation. This could indicate increased barrier permeability, allowing immune cell infiltration into the brain parenchyma.
Previous studies have shown similar findings with age, where the choroid plexus sends inflammatory signals to the brain, resulting in glial activation and reduced cognitive ability.
The current study indicates that "SARS-CoV-2 can infect and inflame brain-barrier cells, including the choroid plexus; and that this barrier—together with peripheral—inflammation is then relayed into the brain parenchyma."
Microglial Activation, Immune Infiltrates
In the study, one subtype of microglia with an activated gene profile was overexpressed in COVID-19, with a significant overlap with gene markers of Alzheimer's disease. These had higher pathologic activation markers and lower immunomodulatory immune checkpoint markers.
This subtype is typically undetectable in similar studies and distinct from those seen in neurodegenerative disease. This could indicate that it is due to increasing CNS inflammation because of inflammation in and across the barrier cells.
Peripheral T cells and macrophages also infiltrated the brain in COVID-19 more highly, in agreement with earlier studies. These findings are absent in patients who died of influenza, indicating it is specific to COVID-19 or to a very few viruses.
A recent study showed that in many neurodegenerative conditions, microglial phagocytosis of living neurons was prominent. This phenomenon, called phagoptosis, occurs in COVID-19, with almost every associated gene marker being upregulated.
Potential Synaptic Dysfunction
Astrocytes are crucial both to homeostasis and to barrier function in the brain. They synapse with neurons to modulate levels of excitatory neurotransmitters like glutamate, using transporters such as EEAT1 (SLC1A3) and EEAT2 (SLC1A2).
Glutamate is required to transmit information rapidly and accurately across synapses so that the brain can process information quickly. As such, it is stored within quick-access presynaptic vesicles.
The researchers found that barrier cells and astrocytes show unregulated repression of glutamate signaling. They observed astrocytic gliosis and antiviral responses, as well as secretion of neurotoxic factors. Both transporters were over-produced, while neuronal glutamate and potassium were lowered, thus reducing neuronal excitability.
Synaptic transmission depends largely on SNARE proteins. Low SNARE expression results in impaired neuronal function due to over-excitation and extra-synaptic spillover of the glutamate outside the synapse. On the other hand, excessive expression reduces the capability for long-term potentiation (LTP) and synaptic plasticity.
SNARE-encoding mRNAs are reduced in COVID-19, as in many neurological conditions, affecting synaptic vesicle function and viability. This accounts for the synaptic dysfunction observed in second/third layer excitatory neurons. These are the thickest layers of the six neocortical strata and command cognitive and other intellectual functions.
Any reduction in firing of the excitatory neurons here is likely to impair synaptic transmission. The firing rate is already low to allow associative learning via a basic and dependable pattern. Despite the involvement of excitatory synaptic transmission, no deficits were observed in COVID-19 patients with clinical neurological features or detectable virus compared to others.
Associations with Chronic CNS Disease
Recently, many cases of long-haul symptoms and signs relating to COVID-19 are being reported to occur after recovery. The researchers attempted to ferret out the reason by comparing DEGs for each type of cell in COVID-19 survivors and individuals with chronic neurological disease, such as Alzheimer's disease, multiple sclerosis, Huntington's disease, and autism spectrum disorder.
The presence of a strong overlap for astrocytes and excitatory neurons may explain this and indicates the relevance of knowledge and treatment of the latter conditions for COVID-19.
Genome-wide association studies (GWAS) also showed that the DEGs in COVID-19 was common to many other neuropsychiatric traits and disorders, including mental disorders like schizophrenia and depression, and cognitive traits.
Implications and Future Directions
The researchers postulate that SARS-CoV-2 infection of the blood-brain barrier cells causes inflammatory signaling and increased permeability to systemic inflammatory signals. The inflamed cortical parenchyma brain is infiltrated by immune cells, with phagoptosis and dysregulated brain homeostasis from astrocyte disruption. The outcome is impaired neuronal function.
This is the first non-invasive snRNA sequencing study to examine the association between neurological changes in COVID-19 in terms of affected pathways and cell types and the changes in other chronic neurological conditions. Such studies could shape treatment strategies.
The interactive data server will be fed with future findings to advance this research. Further research is mandatory to identify correlations between detectable virus or DEGs in the brain and neurological changes. Larger and more representative studies will help determine the generalizability of these findings and the temporal dynamics of these associations.
This study reveals crucial changes in the blood-brain barrier cells, which are typically absent in organoid cultures, which could make the relevance of earlier snRNA-sequencing experiments suspect.
The authors conclude, "Our results nominate COVID-19 pathologic processes in microglia, astrocytes, and neurons not seen in terminal influenza infection. The particular vulnerability of layer 2/3 excitatory neurons in COVID-19 provides a consistent molecular hypothesis for emerging neurological complaints."

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Yang, A. C. et al. (2020). Broad Transcriptional Dysregulation of Brain and Choroid Plexus Cell Types With COVID-19. bioRxiv preprint. doi: https://doi.org/10.1101/2020.10.22.349415. https://www.biorxiv.org/content/10.1101/2020.10.22.349415v1
- Peer reviewed and published scientific report.
Yang, Andrew C., Fabian Kern, Patricia M. Losada, Maayan R. Agam, Christina A. Maat, Georges P. Schmartz, Tobias Fehlmann, et al. 2021. “Dysregulation of Brain and Choroid Plexus Cell Types in Severe COVID-19.” Nature, June. https://doi.org/10.1038/s41586-021-03710-0. https://www.nature.com/articles/s41586-021-03710-0.