Researchers are racing towards the release of potent vaccines and antivirals to counter the ongoing COVID-19 pandemic. One highly promising antiviral approach consists of monoclonal antibodies (mAbs). A recent study published in the preprint server bioRxiv* in October 2020 describes a reliable animal model to demonstrate the powerful capacity of human neutralizing mAbs directed against SARS-CoV-2 to prevent viral binding the host cell receptor and thus block infection. This indicates the lifesaving value of mAbs in severe COVID-19.
The potential for mAbs in preventing and treating viral infections, as shown with Ebola, HIV, MERS- and SARS-CoV, is being widely explored in the current COVID-19 pandemic. SARS-CoV-2 binds to the human angiotensin-converting enzyme 2 (ACE2) receptor via its receptor-binding domain (RBD) located in the spike protein's S1 subunit.
Using the Right Model and Antibody
The researchers from the Israel Institute for Biological Research earlier screened a library of antibodies from infected individuals to develop a collection of human neutralizing antibodies (nAbs) against the SARS-CoV-2 RBD. The current study chose the most potent nAb, MD65, to assess its potential to prevent and treat an infection with this virus when used post-exposure.
For neutralization efficiency and pathogenicity studies, they chose a mouse model that develops human ACE2 and that rapidly develops a clinical phenotype of severe COVID-19. With a high viral load in the lungs, heart, brain, and spleen, 75% of the animals die following infection. This model is, therefore, able to "fatefully recapitulate the SARS-CoV-2 infection and consequently to serve as a reliable model to predict the efficacy of therapeutic strategies.”
Earlier studies only examined the possibility of neutralization by mAbs administered before the viral challenge, when the viral inoculum was at non-lethal dosage.
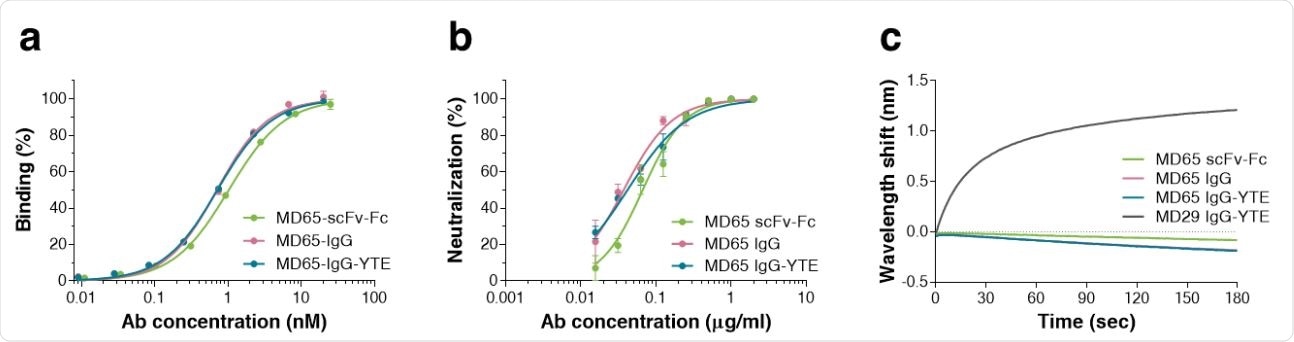
Characterization of the MD65 Ab versions. a Binding profiles of MD65 Ab variants, tested by ELISA against S1. Values along the curve, depict averages of triplicates ± SEM. b SARS-CoV-2 in vitro neutralization potency of the MD65 Ab variants evaluated by plaque reduction neutralization test (PRNT). Values are averages of triplicates ± SEM. c BLIdetermined binding of hACE2 to RBD in the presence of MD65 Ab variants or MD29 IgGYTE (as a control). Each of the biotinylated antibodies was immobilized on a streptavidin sensor, saturated with RBD, washed and incubated with recombinant hACE2 for 180 sec. Time 0 represents the binding of the hACE2 to the antibody-RBD complex.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The MD65 Antibody
MD65 is a recombinant single-chain human-Fc (scFc) molecule. To test its value as a potential therapeutic, the researchers used three variants: the full IgG recombinant form, the sc-Fc form, and the IgG form with a triple YTE mutation in the Fc region that enhances the binding affinity for the human FcRn31 residue. This allows its persistence in serum for a longer time, as required of a therapeutic molecule.
The investigators evaluated three forms of the MD65 molecule (scFv-Fc, IgG, and IgG-YTE). They found that all three showed comparably potent specific binding and neutralization efficiency. The IgG forms had a slightly better affinity compared to the earlier sc-Fc form.
All three blocked RBD-ACE2 binding, but the IgG slightly better than the other. This could mean that affinity is related to neutralizing capacity for SARS-CoV-2, but more study is required.
Long Half-Life of MD65 IgG-YTE
The MD65-IgG-YTE format was shown to have a much higher affinity, following its modification, with the rate of the 'on' conformation being threefold higher and the 'off' rate being much lower. In fact, this IgG-YTE version bound the human FcRn ten times better at low pH, compared to the IgG form.
Following intravenous (IV) or intraperitoneal (IP) MD65 IgG-YTE antibody, the plasma half-life was about 3 hours.
SARS-CoV-2 Infection Blocked by MD65 IgG-YTE
The researchers showed that mice inoculated with a lethal dose of the virus lost weight from 5 days after the infection, and 75% of them died within 6-9 days. In the current experiment, infected mice were treated by two doses of mAb administration 4 days apart, given IP, to enable a steady therapeutic concentration of the antibody over this period.
Mice given MD65 4 hours before viral inoculation remained completely normal. In contrast, when a similar Fc molecule that did not target the RBD was used, lethal infection resulted.
Secondly, they administered MD65 at 1, 2, 3, or 4 days post-infection (dpi). They found that at 1 and 2 dpi, it prevented weight loss and death in 100% of treated animals. Even at 3 dpi, 100% of treated mice survived, albeit with some weight loss over the 10 days of observation.
When treated at 4 dpi, 60% of the animals lived, while the others died within 6-9 days.
The researchers describe their findings: “Taken together, these results demonstrate, for the first time, the therapeutic value of human monoclonal antibodies as a lifesaving treatment of severe and lethal COVID-19 infection model.”
Seroconversion in MD65 IgG-YTE -Treated Animals
Earlier studies showed seroconversion after antibody administration. That is, the treated animals who survived infection had specific antibodies and resisted re-infection. The researchers measured the anti- SARS-CoV-2 Spike antibody content in the plasma of infected and treated mice at 14 dpi in the current experiment.
They found that when pretreatment with the MD65 Ab prevented seroconversion, all of the inoculum viruses were immediately neutralized. When treated with MD65 at 1 or 2 dpi, the mice showed a significant antibody response targeting the spike S1 unit and the RBD. This indicates the antibody response contains partly or fully neutralizing antibodies, thus protecting the host against re-infection.
Further studies will need to measure the neutralization titer of such antibodies, which was not done in the present experiment because of the confounding effect of the administered MD65 Ab.
There was no evidence of antibody-dependent enhancement (ADE) of infection, despite the post-exposure treatment of animals following established infection, which could theoretically lead to immune complex formation.
Conclusion
The study concludes, “The data demonstrates for the first time, to the best of our knowledge, high-effective post-exposure therapy of SARS-CoV-2 lethal infection in an animal model using a fully human monoclonal antibody. This antibody extends the therapeutic window permitting initiation of lifesaving treatment at late stages post-infection.”
This demonstrates the value of the MD65 antibody as a sound basis for developing a future human antibody.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources