The coronavirus disease (COVID-19) continues to spread across the globe, with many countries reporting a second wave of the outbreak. Caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the disease dramatically affects the elderly and those with underlying medical conditions. A vaccine to protect against the virus is needed now more than ever.
One of the forerunners in developing a COVID-19 vaccine is the University of Oxford, in partnership with AstraZeneca. Together, they developed the ChAdOx1 nCoV-19 vaccine.
Now, a new study published on the pre-print server bioRxiv* shows that a single dose of the vaccine has induced cellular and humoral immunity in aged or older mice. A second dosage was needed to enhance the vaccine's immune response, showing that a prime-boost strategy may be an essential step to boost immunogenicity in the elderly.
The vaccine
The University of Oxford Jenner Institute, along with AstraZeneca, developed the ChAdOx1 nCoV-19 vaccine. It uses a replication-deficiency chimpanzee adenovirus to provide a SARS-CoV-2 protein to induce a protective immune response.
ChAdOx1 has been used in the past to develop investigational vaccines against many pathogens, including the Middle East respiratory syndrome coronavirus (MERS-CoV), a close relative of the SARS-CoV-2.
The team from the Babraham Institute, the Jenner Institute, and the Pirbright Institute used this technology to develop the vaccine against SARS-CoV-2 as soon as the first cases of COVID-19 emerged.
Vaccines are crucial in the battle against COVID-19. Though candidate vaccines are now in the last phase of human trials, it is essential to know that age-related changes in the immune system may hamper the production of an immune response after vaccination. As the elderly are at a high risk of infection with SARS-CoV-2, a considerable effort has been made to develop vaccines to be more effective in those who are more than 65 years old.
"Modifications to vaccines include increasing the antigen dose and the use of adjuvants, which can support the enhancement of immune responses in older people compared to standard formulations of vaccines," the team explained.
"This indicates that the success of a SARS-CoV-2 vaccine may require alterations to the dosing regimen, including an increase in the dose or the number of doses in order to induce a sufficient immune response in older people," they added.
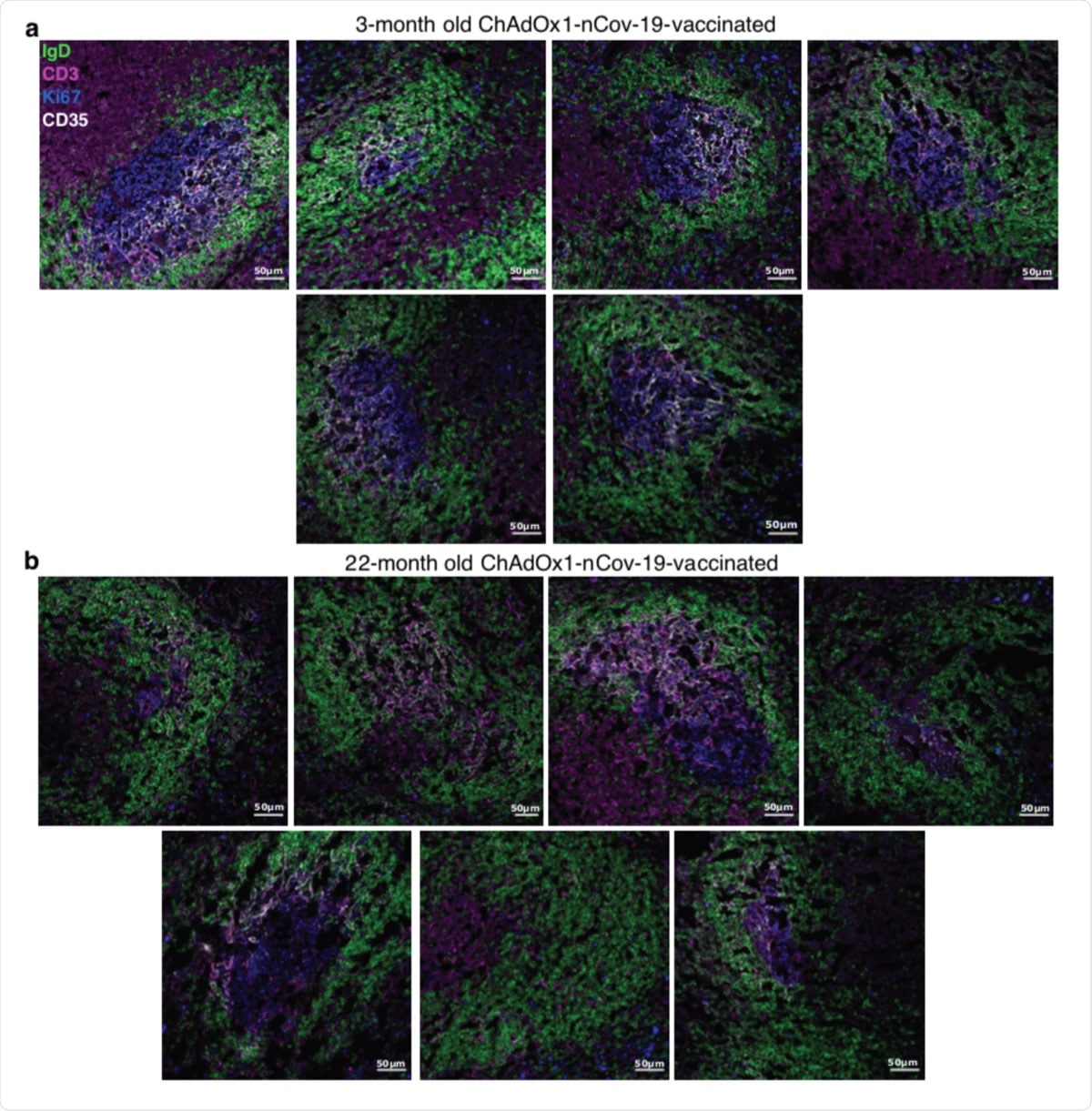
Germinal center images nine days after ChAdOx1 nCoV-19 immunization in adult and aged mice. Confocal images of the spleen of ChAdOx1 nCoV-19 immunised 3-month-old (a) and 22-month-old (b) mice. 10 μm spleen sections were stained with anti-IgD (green), anti-CD3 (pink), anti-Ki67 (blue) and anti-CD35 (white) antibodies. Each image is from a different mouse. The scale bars indicate 50μm.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The study
A vaccine's efficacy in older adults can be determined through trials. Apart from trials, studies in aged animals can be used to test alternative strategies for vaccination. The current study will help in developing an effective vaccine for older adults.
The researchers demonstrated that a single dose of the vaccine had induced a B and T cell response in three-month-old mice. There was an increase in humoral immunity, which was combined with the formation of polyfunctional vaccine-specific antibodies.
In mice that are older at about 22 months, a single dose of the vaccine has led to the formation of Th1 cells and other antibodies. The team noticed that the cellular and humoral response declined in the 22-month-old mice compared to the 3-month-old adult mice.
Injection with a second dose of the vaccine enhanced the germinal center response and antibody titer in older mice. Overall, the study shows that the efficacy of ChAdOx1 NCoV-19 can be enhanced.
"Together, this indicates that the immunogenicity of ChAdOx1 nCoV-19 can be enhanced in older individuals through the use of a prime-boost vaccination strategy," the team said.
Findings
The current pandemic has significantly impacted economies and societies. Since older people are susceptible to have severe health outcomes caused by COVID-19, a vaccination strategy to protect this age group is essential.
The researchers have found that the ChAdOx1 nCoV-19 vaccine induced an immune response in older mice models, but it has a reduced magnitude compared to that seen in younger mice, consistent with other immunization studies.
The study also showed that a second dosage or a booster at the same dose could enhance B cell, helper T cell, and C8+ T cell response in older mice.
"We report that a second dose enhances the immune response to this vaccine in aged mice, indicating that a prime-boost strategy may be a rational approach to enhance immunogenicity in older persons," the researchers wrote in the paper.
Hence, it is vital to consider the dosage of the vaccine in human trials, specifically tailoring it to the patient's age. This way, many people from all walks of life receive protection against the coronavirus disease, which has now infected over 44 million people and has claimed the lives of at least 1.18 million people.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Source:
Journal references:
- Preliminary scientific report.
Silva-Cayetano, A., Innocentin, S., Foster, W. et al. (2020). A booster dose enhances the immunogenicity of the COVID-19 vaccine candidate ChAdOx1 nCoV-19 in aged mice. bioRxiv. https://www.biorxiv.org/content/10.1101/2020.10.27.357426v1
- Peer reviewed and published scientific report.
Silva-Cayetano, Alyssa, William S. Foster, Silvia Innocentin, Sandra Belij-Rammerstorfer, Alexandra J. Spencer, Oliver T. Burton, Sigrid Fra-Bidó, et al. 2021. “A Booster Dose Enhances Immunogenicity of the COVID-19 Vaccine Candidate ChAdOx1 NCoV-19 in Aged Mice.” Med 2 (3): 243-262.e8. https://doi.org/10.1016/j.medj.2020.12.006. https://www.cell.com/med/fulltext/S2666-6340(20)30034-9?.