A recent study by US researchers reveals that brilacidin, a small synthetic molecule with peptide-like properties, exerts potent in vitro antiviral activity against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – both as a standalone treatment and in combination with FDA-approved remdesivir. The study is currently available on the bioRxiv* preprint server.
SARS-Cov-2, the pathogen and causative agent of coronavirus disease 19 (COVID-19), has been responsible for more than 1.2 million deaths worldwide since it emerged at the end of 2019. So far, there are no vaccines approved for preventing SARS-CoV-2 infection, and all presently available treatments are only modestly effective.
Therefore, while the quest for a vaccine is developing at an unprecedented pace, there is an urgent global need for therapeutic strategies to safely treat COVID-19 and reduce its substantial morbidity and mortality.
This is where natural and synthetic antimicrobial peptides come into play, also known as Host Defense Proteins/Peptides (HDPs), since they have confirmed inhibitory activity against multiple viruses. These compounds selectively and multifariously interact with different pathogens, increasing their vulnerability to proteolysis and degradation.
Within the broad context of the global COVID-19 pandemic and the possible therapeutic role of HDPs, a US-based research group - from George Mason University, the State University of New Jersey and Innovation Pharmaceuticals Inc. - evaluated de novo designed brilacidin in laboratory settings to appraise whether the drug might exhibit antiviral properties against SARS-CoV-2.
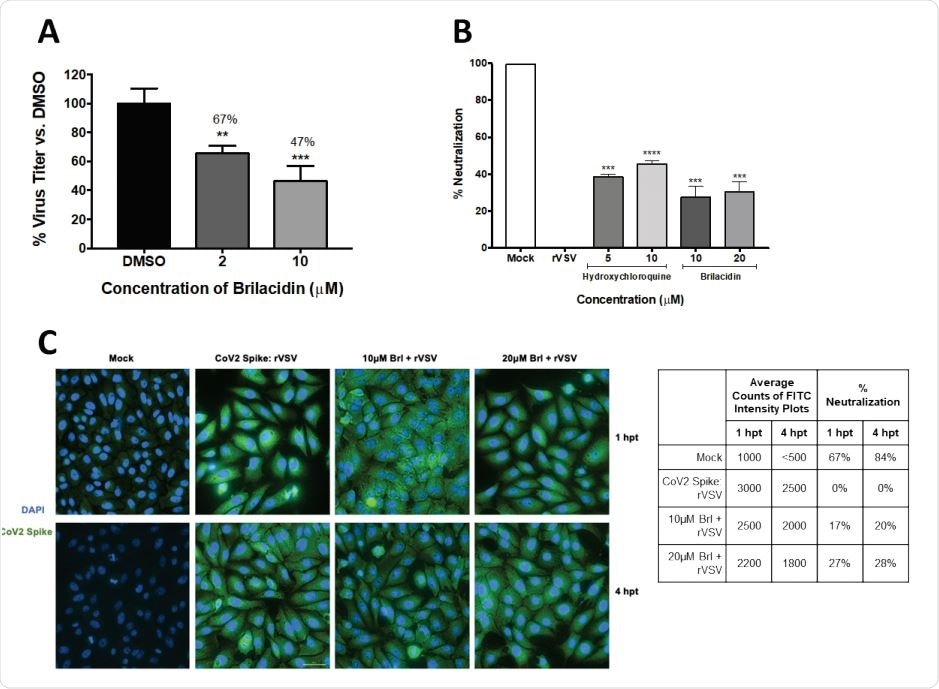
Brilacidin inhibits SARS-CoV-2 replication (Vero cells) (A) Vero cells were pretreated for 2h with 2 or 10μM brilacidin, infected with SARS-CoV-2 non-directly at MOI 0.1 for 1h, and post-treated with media containing brilacidin as described in Materials and Methods. At 16hpi, viral supernatants were evaluated by plaque assay as described in Materials and Methods. Figure 2B, 2C. Brilacidin appears to impact entry of SARS-CoV-2 (Vero cells) (B) Brilacidin was measured at 10 and 20μM for neutralization activity against a luciferase-expressing pseudotyped virus (rVSV) containing the SARS-CoV-2 spike protein using luciferase assay in Vero cells at 24hpt as described in Materials and Methods and compared to neutralization activity of hydroxychloroquine. (C) Vero cells were treated with 10 or 20μM brilacidin for neutralization activity against SARS-CoV-2 rVSV, and cells imaged and quantified using fluorescent microscopy and FITC surface intensity plots at 1 and 4hpt as described in Materials and Methods. Graphs are representative of one independent experiment performed in technical triplicates (n=3). Brl indicates brilacidin. **p<0.0021, ***p<0.0002, ****p<0.0001.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Cell lines and inhibition assays
The potential antiviral activity of brilacidin against SARS-CoV-2 was initially assessed with the use of Vero cells as an infection model. Toxicity assessment of brilacidin in these cells was performed by incubating them with increasing concentrations of the compound for 24 hours, and the treatment effect was appraised by utilizing plaque assay.
Moreover, the potential of brilacidin for interfering with viral entry was also assessed in the Vero cell line by observing the angiotensin-converting enzyme 2 (ACE2) spike glycoprotein interaction. This was done using a recombinant vesicular stomatitis virus (rVSV)-pseudotyped SARS-CoV-2 expressing a luciferase reporter gene.
The aforementioned approach was supported with experiments conducted in the Calu-3 infection model to confirm that brilacidin can prompt anti-SARS-CoV-2 activity in an ACE2-positive human lung cell line.
To independently appraise the impact of brilacidin on viral particles and, hence, add support to the role of brilacidin as an inhibitor of viral entry, a direct virus inhibition assay was conducted alongside virus neutralization observed in the presence of antibodies.
Early-stage SARS-CoV-2 inhibition
“Our experiments in the Vero cell line model demonstrate brilacidin decreases viral load in a robust manner when the virus is pre-incubated with brilacidin, suggesting brilacidin impacts the virus directly,” say the study’s authors.
More specifically, after brilacidin treatment, a dose-dependent decrease in infectious viral titer was observed, with a maximum of 53% viral inhibition in the presence of the higher drug concentration (i.e., 10 micromoles). Likewise, the drug also achieved a high selectivity index.
The direct impact observation is supported by the inhibition observed in the context of a replication-incompetent pseudovirus, which additionally shows the propensity of brilacidin to exert inhibitory activity during the early stages of viral infection, but also to impede the interaction between SARS-CoV-2 spike glycoprotein and ACE2 receptor on the cells.
Furthermore, all experiments that were conducted in the aforementioned Vero and Calu-3 cell line models were supportive of an early inhibition exerted by brilacidin on the virus – indicating, in turn, the compound’s considerable impact on viral integrity.
A potential broad-spectrum coronavirus inhibitor
“Collectively, our data demonstrate that brilacidin exerts potent inhibition of SARS-CoV-2 and thus supports brilacidin as a promising COVID-19 drug candidate”, concludes study authors in this bioRxiv paper.
Their data suggest that SARS-CoV-2 inhibition in used cell culture models primarily arose as a result of the impact of brilacidin on viral entry and subsequent disruption of viral membrane viral integrity. Brilacidin has also demonstrated synergistic antiviral activity when combined with remdesivir, currently the only FDA-approved treatment for COVID-19.
In any case, more detailed mechanistic studies are planned, while experiments using endemic human coronaviruses are ongoing. On top of that, additional testing planned in other highly lethal coronaviruses (i.e., SARS-CoV and MERS-CoV) will fully gauge the potential of brilacidin as a broad-spectrum coronavirus inhibitor.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Bakovic, A. et al. (2020). Brilacidin, a COVID-19 Drug Candidate, Exhibits Potent In Vitro Antiviral Activity Against SARS-CoV-2. bioRxiv. https://doi.org/10.1101/2020.10.29.352450, https://www.biorxiv.org/content/10.1101/2020.10.29.352450v1
- Peer reviewed and published scientific report.
Bakovic, Allison, Kenneth Risner, Nishank Bhalla, Farhang Alem, Theresa L. Chang, Warren K. Weston, Jane A. Harness, and Aarthi Narayanan. 2021. “Brilacidin Demonstrates Inhibition of SARS-CoV-2 in Cell Culture.” Viruses 13 (2): 271. https://doi.org/10.3390/v13020271. https://www.mdpi.com/1999-4915/13/2/271.