The coronavirus disease (COVID-19) is a respiratory illness that affects the lungs. The most common symptoms associated with the infection include fever, a continuous dry cough, and difficulty breathing (dyspnea or shortness of breath). In more severe cases, the illness causes viral pneumonia. In more critical cases, it can eventually lead to acute respiratory distress syndrome (ARDS), which can often be fatal.
As the COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) agent, has evolved, more evidence is coming to light that the virus can also affect other organs in the body, including the heart and brain.
An illuminating new study conducted by researchers at the Washington University School of Medicine, the University of Wisconsin Hospital, Baylor College of Medicine, Vanderbilt University, Creighton University the University of Texas Medical Branch, The Jackson Laboratory for Genomic Medicine, USA and the University of Heidelberg, Germany, shows that SARS-CoV-2 can infect heart cells in a lab dish (in vitro), indicating it may be possible for heart cells in COVID-19 patients to be directly infected by the virus.
The study
Recent studies on COVID-19 have shown evidence of cardiac involvement, showing that myocardial injury and myocarditis are predictors of poor outcomes in severely ill patients. Nevertheless, little is known regarding SARS-CoV-2 tropism within the heart and whether cardiac complications stem from myocardial infection.
To arrive at the study’s findings, the researchers developed a human-engineered heart tissue (EHT) model. They tested the hypothesis that SARS-CoV-2 induces heart pathology by infecting heart cells and activating immune responses.
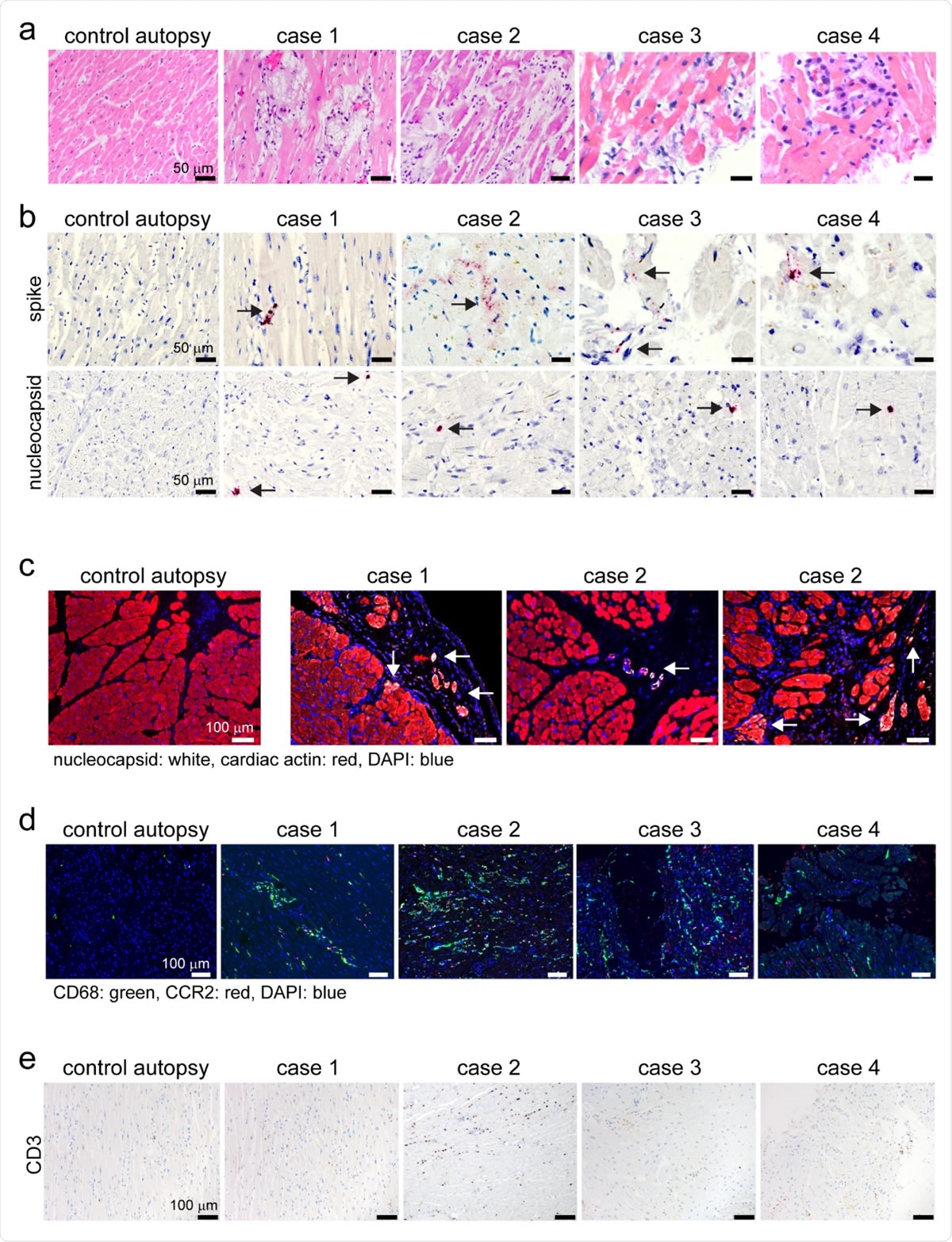
Human autopsy and endomyocardial tissue from patients with suspected COVID- 19 myocarditis show evidence of SARS-CoV-2 cardiomyocyte infection. a, Hematoxylin and eosin staining of cardiac autopsy (anterior left ventricular wall), and biopsy samples (right ventricular septum) from subjects without COVID-19 (control case) and patients with a clinical diagnosis of COVID-19 myocarditis (case 1-4). b, In situ hybridization of cardiac autopsy and biopsy tissue for SARS-CoV-2 spike and nucleocapsid RNA (red) showing evidence of viral infection. Hematoxylin: blue. Arrows denote viral RNA staining in cells with cardiomyocyte morphology. c, Immunostaining of control and COVID-19 myocarditis cardiac autopsy tissue for SARS-CoV-2 nucleocapsid (white) and cardiac actin (red). DAPI: blue. Arrows denote nucleocapsid staining in cardiomyocytes .d, Immunostaining of control and COVID-19 myocarditis cardiac autopsy and biopsy tissue for CD68 (green) and CCR2 (red). DAPI: blue. e, Immunostaining of control and COVID-19 myocarditis cardiac autopsy and biopsy tissue for CD3 (brown). Hematoxylin: blue.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Study findings
The team showed that the virus selectively infects and replicates within hPSC-derived cardiomyocytes, resulting in cell death. Based on the study’s findings, the team noted that cardiomyocyte or heart cell infection is dependent on the angiotensin-converting enzyme 2 (ACE2) expression and endosomal cysteine protease activity.
In the study, which appeared on the preprint bioRxiv* server, the researchers revealed that SARS-CoV-2-infected EHTs manifested the typical characteristics of myocarditis or heart inflammation. These include the stimulation of immune cells, heart cell death, and decreased contractile force generation.
The team also noted that the autopsy and biopsy samples from four patients who tested positive for COVID-19 and myocarditis exhibited patchy cardiomyocyte infection, along with myocardial cell death and the accumulation of macrophages, which are specialized cells involved in the detection, phagocytosis, and destruction of bacteria and other harmful organisms.
The team also explored if human heart cells may be susceptible to SARS-CoV-2 infection. They studied the expression of the angiotensin-converting enzyme 2 (ACE2) within the human heart. Past studies have shown that ACE2 acts as a cell-surface receptor for SARS-CoV-2 and binds with the virus spike protein (sometimes referred to as its S-protein) in many human cell types. The team unveiled that cardiomyocytes express ACE2 and they showed a significant variation in ACE2 expression between cardiomyocytes.
“We provide evidence that SARS-CoV-2 readily infects and replicates within human cardiomyocytes, indicating that viral infection likely contributes to the pathogenesis of COVID-19 myocarditis,” the team explained.
The team believes that engineered heart tissue has paved the way to gain better insights into the link between heart cell infection, myocardial inflammation, and contractile dysfunction. Also, EHTS can help scientists determine the various effects of COVID-19 on the human heart, helping clinicians provide better interventions and reduce mortality among patients.
“We provide evidence that human EHTs recapitulate many features of COVID-19 myocarditis, demonstrate that SARS-CoV-2 infection of EHTs can produce multiscale changes spanning from the molecular to functional levels, and show that EHTs serve as useful tools for dissecting mechanisms that contribute to cardiac pathology,” the team concluded.
The coronavirus pandemic continues to ravage across the globe. Overall, there are over 50 million confirmed cases, and at least 1.25 million deaths. The United States remains the country with the highest number of cases, reaching 9.96 million. India and Brazil follow with a staggering 8.5 million and 5.66 million cases, respectively.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Source:
Journal references: