Considerable evidence has accumulated to suggest that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus may spread through prolonged or brief contact with infected patients - with the infection being spread through respiratory droplets and aerosols. The need to understand how these infectious droplets behave becomes ever more urgent. A recent study published in the preprint server medRxiv* in October 2020 reports the results of such an investigation.
It is known that the environmental conditions surrounding a liquid droplet affect the droplet’s growth or shrinkage, but quantitative information on the behavior of such a droplet originating from a puff of breath is still missing. The current study aims to address this deficiency and thus help to understand how the disease is transmitted via airborne particles.
The virus is thought to be carried in millions of micrometer-sized minute droplets of saliva and mucus. These are expelled from the mouth during ordinary breathing but are believed to expel at a much higher intensity during singing, sneezing, shouting, coughing, or speaking.
However, many factors can affect the airborne transmission, and besides a clinical perspective, fluid mechanics is also important to understand how disease spreads through airborne droplets.
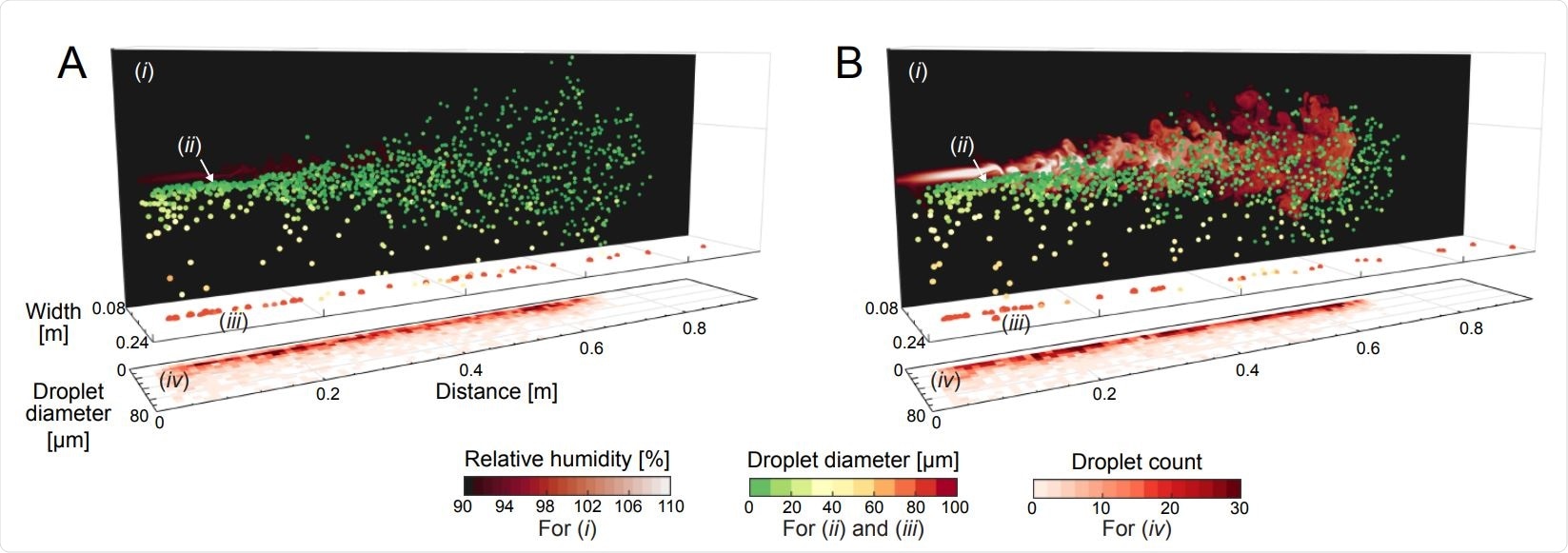
Flow visualization snapshots from our direct numerical simulations of water droplets in a warm humid puff in ambient air at (A) θamb = 30◦C and (B) θamb = 10◦C, both at RHamb = 90%. The snapshots show (i) vertical 2D planes of the local RH fields, (ii) the instantaneous droplets spatial distribution, (iii) the heavy large droplets which already fell on the ground, and (iv) the instantaneous droplet size histograms versus distance. The local RH planes are taken from the vertical mid-plane of the puff and are plotted on the background for clarity. Droplets are color coded by their instantaneous sizes. Initial droplet sizes are prescribed with a distribution similar to J. P. Duguid (13) and are injected evenly in time with the same local inflow velocity. The initial temperature of the droplets and puff is 34◦C. Both snapshots are taken at 0.6s corresponding to the cut-off time of the puff. In the colder conditions of (B), the expelled humid puff over-saturates, which in turn dictates growth of smaller droplets caught within the puff. Correspondingly, the droplet counts are confined within a narrower range of sizes in (B) as compared to (A)
Simulation of Droplets in Turbulent Vapor Puff
The researchers used direct numerical simulations (DNS) to model a puff of respiratory air in turbulent conditions. They also included the possibilities of exchange of mass and heat with the surrounding air. The particular focus was to identify how these droplets act when exposed to variations in ambient temperature and relative humidity (RH), using physical flow principles in the form of Lagrangian statistics.
Early work in the 1940s suggested that droplets expelled from the mouth undergo gravity-dependent settling and then slowly evaporate. Thus, their surface area reduces over time. This gave rise to a simple framework using only the ambient temperature and RH to predict the droplet lifetime, based on the so-called d-2 law.
However, this was shown to be insufficient in predicting the actual dynamics of a particle. The flow of such droplets may undergo a jet-like pattern, as with speaking, spreading the droplet nuclei within enclosed spaces.
Again, a turbulent puff of air expelled from the lungs during respiration is more realistic a simulation of actual events than the consideration of a single droplet. Using this concept in DNS, droplets were shown to survive 100 times longer in such a puff with high RH than the d-2 law estimate shows.
Droplet Growth due to Supersaturation
In the current study, the researchers found that respiratory droplets grow at first before they begin to shrink by evaporation. When the droplet leaves the mouth and enters cold ambient air with high RH, the inability of this air to hold water vapor causes supersaturation of the turbulent puff of vapor to occur, and the droplet grows. This growth is dependent on certain ambient conditions and is greater when the ambient temperature is lower and the RH higher.
As a person speaks for a long time, the warm air ejected from the mouth into the cold air maintains a zone of supersaturation for a prolonged duration, protecting the droplets. Thus, when the air is cold and humid, these tiny droplets are able to move to a greater distance, and thus the infection can be propagated more easily.
The investigators also constructed a theoretical model that predicts the actual RH at the level of the droplets, as the droplet moves away from the mouth. They also determined the parameters for ambient temperature and RH at which such supersaturation occurs.
They found a high degree of agreement with the simulation. They say, “Our theoretical model can also be applied to other respiratory events whenever there is jet-like transport, such as speaking, which is extremely relevant for asymptomatic and presymptomatic spreading of the coronavirus.”
Implications
The study summarizes, “Our finding implies that tiny droplets of the size of micrometers can propagate further away when the weather is cold and humid due to the protection from the super-saturated vapor field. Our results culminate in a temperature-RH map that can be employed as an indicator for droplet growth or shrinkage.”
The researchers say, despite the utility of such simulations for proposing better public health guidelines and the need for better indoor ventilation, when confronted by varying weather conditions and seasonal climate changes, it is still necessary to study the droplets and droplet nuclei themselves for the presence of infectious virus even after evaporation. The conditions under which people gather in close contact for long enough to allow such infection to spread, and determining the dose of live virus that can spread the infection are other issues. These need to be determined by other means than fluid mechanics.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources