The coronavirus disease 2019 (COVID-19) pandemic has impacted over 50 million people so far and has claimed more than 1.25 million lives globally. As the virus continues to spread, with many countries experiencing second waves right now, we still do not have an effective vaccine against COVID-19. This makes finding an effective drug to treat the disease very important, and scientists worldwide are racing against time to find therapeutic solutions that will help treat COVID-19 patients.
Keeping this in mind, a team of researchers from various institutes across Pakistan, Chile, Canada, and the USA recently conducted a clinical trial to investigate the potential effectiveness of a combination of honey and Nigella sativa (HNS) in treating COVID-19 patients. Their study is published on the preprint server medRxiv* prior to the scientific peer review process*. Since previous studies show that both components of HNS have proven anti-microbial, anti-viral, anti-inflammatory, and immune-modulatory effects, the researchers wanted to assess the efficacy of HNS in fighting COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Healing properties of honey and Nigella sativa
Honey has been reported to have beneficial effects against many viruses, including herpes simplex virus (HSV), rubella virus, Hepatitis virus, and Varicella Zoster virus. It is also effective against many multidrug-resistant bacterial strains, especially when used along with antibiotics. In addition to its immunity-boosting effects that stimulate innate as well as adaptive immune responses, honey has been shown to be beneficial in fighting upper respiratory tract infections.
Nigella sativa is a medicinal plant commonly known as Black Cumin and has been proven to have anti-viral properties against many viruses, including mouse cytomegalovirus and HCV. In vitro studies have shown that it can decrease the replication of severe acute respiratory syndrome coronavirus (SARS-CoV). Some of its components have a high affinity to many SARS-CoV-2 proteins and enzymes.
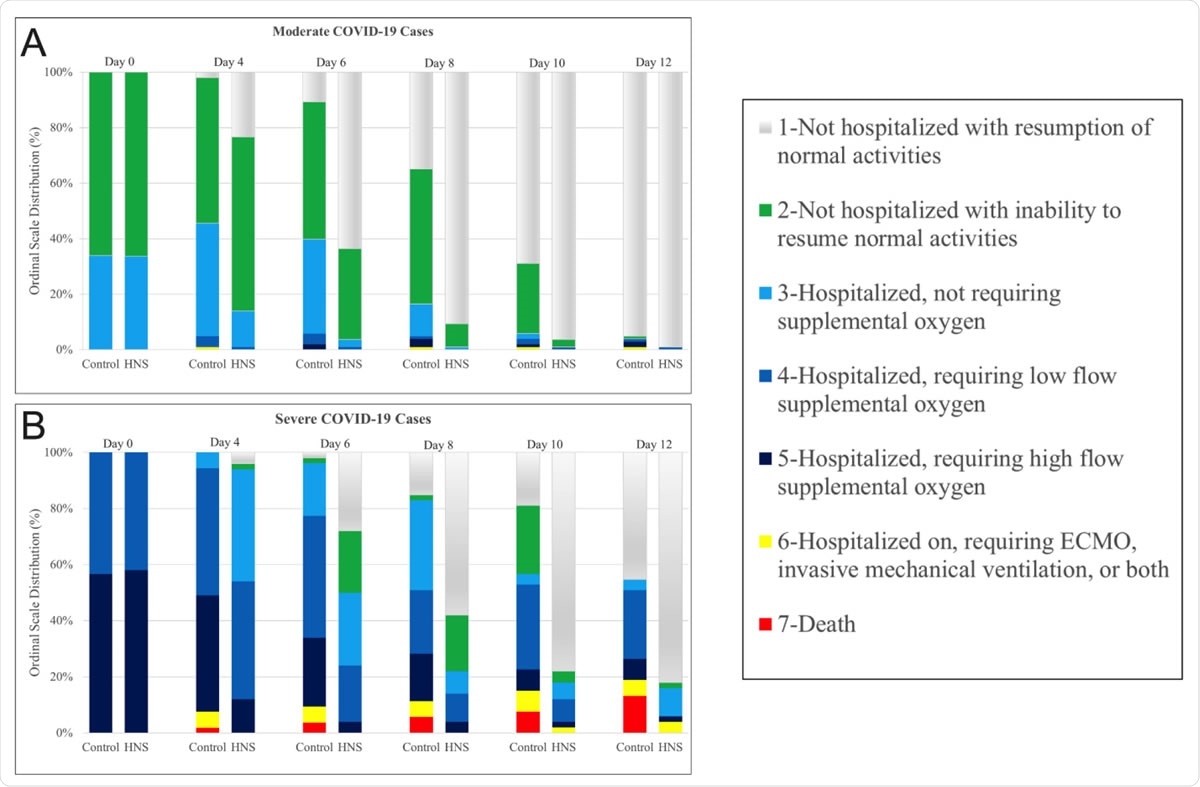
Kinetics of clinical status grading in Ordinal-Scale in COVID-19 patients. The Figure shows kinetic changes in clinical grade score (in 7-point ordinal-scale) in COVID-19 patient receiving the treatment (HNS) or placebo (Control). Note increases numbers of patients within scale 1 in the HNS group both for the moderate and severe cases.
A randomized, controlled trial to study the action of HNS against COVID-19
The researchers performed a multicenter, randomized, controlled trial in patients with COVID-19 of varying severity. Randomized patients received either a combination of 1 gm/kg/day of honey and 80 mg/kg/day of Nigella sativa or placebo for 13 days. Viral clearance, symptoms, alleviation, and 30-day mortality were some of the outcomes.
“The trial results show that the use of HNS in COVID-19 patients promotes viral clearance and reduces the severity of the disease.”
Of the 313 patients, part of the study cohort, 210 patients with moderate symptoms and 103 with severe COVID-19 underwent randomization. Among these patients, 107 received HNS, and 103 moderate cases received a placebo. Fifty severe COVID-19 patients were given HNS, and 53 severe cases received placebo. Administration of HNS led to alleviation of symptoms by day 3 in moderate cases and day 7 in severe cases.
“Anti-diabetic, anti-hypertensive, cardio-protective and broncho-dilatory properties of HNS make it even more beneficial in diabetic, hypertensive, cardiac and asthmatic patients who have a higher COVID-19 associated mortality.”
HNS could be an affordable, home-based, OTC treatment option for COVID-19 patients
The study results show that HNS helped with symptoms alleviation and viral clearance and reduced mortality in patients with moderate and severe disease. According to the team, HNS can be used as a safe and effective therapy in COVID-19 patients as it promotes quicker recovery and survival. Thus, they concluded that HNS represents an affordable therapeutic option and can be used alone or in combination with other therapies to fight COVID-19.
Some benefits of this potential treatment option are its ‘over the counter’ availability, affordability – less than $5 for the entire treatment course, and ease of administration as it can be a home-based remedy. Moreover, HNS can also be used in combination with other drugs for increased efficacy. The authors believe that this treatment will significantly reduce the burden on global health care systems.
“A multinational study with larger sample size is required to investigate potential variations in responses to the treatment in COVID-19 patients from different racial and ethnic origins.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Efficacy of honey and Nigella sativa against COVID-19: HNS-COVID-PK Trial Sohaib Ashraf, Shoaib Ashraf, Muhammad Ahmad Imran, Moneeb Ashraf, Larab Kalsoom, Uzma Nasim Siddiqui, Muhammad Ghufran, Nighat Majeed, Iqra Farooq, Zaighum Habib, Abubakar Hilal, Zain-ul-Abdin, Ayesha Khaqan, Muhammad Kiwan Akram, Sidra Ashraf, Rutaba Akmal, Sundas Rafique, Khawar Nawaz, Shahroze Arshad, Sohail Ahmad, Kanwal Hayat, Ali Arshad, Muhammad Faisal Nadeem, Muhammad Hassan, Abeer-bin-Awais, Muhammad Azam, Muhammad Suhail, Sibgha Zulfiqar, Imran Anwar, Saulat Sarfraz, Ayesha Hamayoun, Amber Malik, Hui Zheng, Talha Mahmood, Mahmood Ayyaz, Ali Ahmad, Muhammad Ashraf, Qazi Abdul Saboor, Mateen Izhar medRxiv 2020.10.30.20217364; doi: https://doi.org/10.1101/2020.10.30.20217364, https://www.medrxiv.org/content/10.1101/2020.10.30.20217364v2
- Peer reviewed and published scientific report.
Ashraf, Sohaib, Shoaib Ashraf, Moneeb Ashraf, Muhammad Ahmad Imran, Larab Kalsoom, Uzma N. Siddiqui, Iqra Farooq, et al. 2022. “Honey and Nigella Sativa against COVID ‐19 in Pakistan ( HNS‐COVID‐PK ): A Multicenter Placebo‐Controlled Randomized Clinical Trial.” Phytotherapy Research 37 (2): 627–44. https://doi.org/10.1002/ptr.7640. https://onlinelibrary.wiley.com/doi/abs/10.1002/ptr.7640.