Immunity is a curious thing. While essential in protecting the body against invading pathogens and foreign antigens, it can also turn against the body and trigger destructive immunological processes. A new study published on the preprint server bioRxiv* in November 2020 reports on the role played by hyperactive macrophages in a range of acute and chronic hyper-inflammatory conditions.
Macrophages are immune defense cells, forming part of the innate immune system that watches for infections. However, their response to pathogens of any sort can trigger chronic or excessive inflammation as well. This kind of abnormal macrophage activation is seen in rheumatoid arthritis (RA) and other autoimmune conditions. One goal of RA treatment is to modulate macrophage activation states and reduce macrophage infiltration into the inflamed tissue.
In the lungs, too, infection can cause macrophage-induced inflammation, which results in the destruction of lung tissue. It can also set off a cytokine storm, seen in acute respiratory distress syndrome (ARDS). Macrophage activation is dependent on receptors, which in turn respond to a variety of external signals. Most of these are mediated by cytokines and antigens present on microbes.
Macrophage activation vs super-activation
One of the best known and most important macrophage activator molecules is interferon-γ (IFN-γ). Macrophages primed by IFN-γ exposure respond more powerfully to subsequent stimulation. Another similar activator class contains Toll-like receptor (TLR) agonists, which prime macrophages such that they secrete molecules that form the inflammatory body called the inflammasome. Activation of inflammasome components causes the cell to die by pyroptosis along with the release of interleukin -1β (IL-1β).
In the words of the researchers, “An important component of the macrophage response to a primary signal is upregulation of a secondary super-activator receptor that can then transform these primed macrophages into an explosive, potentially pathogenic inflammatory state.”
The researchers explored several inflammatory conditions such as RA, Crohn’s disease and severe COVID-19, finding a novel secondary super-activating macrophage receptor called SLAMF7. This, they say, is key to the pathogenesis of these conditions.
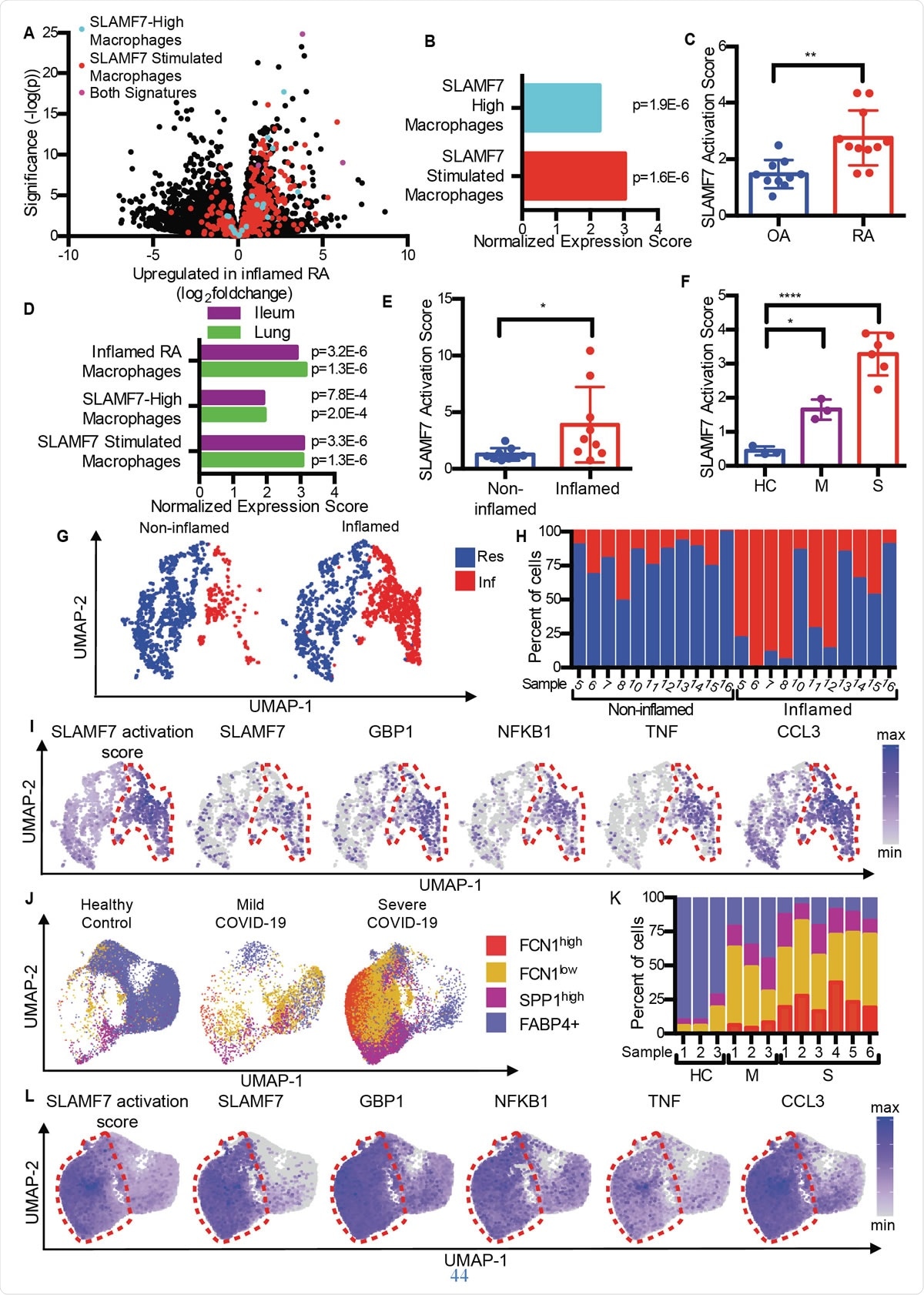
SLAMF7 super-activated macrophages drive inflammation in autoimmune and infectious disease. A) Volcano plot from Fig. 1A highlighting genes from the “SLAMF7-High Macrophage Signature,” the “Macrophage SLAMF7 Stimulation Signature,” and genes included in both signatures. B) Gene set enrichment analysis comparing differential gene expression in RA versus OA to the “SLAMF7-High Macrophage Signature” and “Macrophage SLAMF7 Stimulation Signature.” C) SLAMF7 activation score for bulk RNA-seq data on synovial macrophages from patients with OA (n=10) or RA (n=11). Data represent mean ± SD. D) Gene set enrichment analysis comparing gene expression from macrophages from inflamed ileal tissues in patients with Crohn’s disease or lungs of patients with COVID-19 with the “Inflamed RA Macrophage Signature”, the “SLAMF7-High Macrophage Signature” and the “Macrophage SLAMF7 Stimulation Signature.” E) SLAMF7 activation score for macrophages from noninflamed (n=9) and inflamed ileal tissues (n=9). F) SLAMF7 activation score for bronchoalveolar lavage macrophages from healthy controls (n=3), or individuals with mild (n=3) or severe COVID-19 (n=6). Data in E-F represent mean ± SD. G) UMAP plot of macrophage clusters from involved and uninvolved ileal tissues. H) Percent of macrophages from each donor assigned to each cluster. I) UMAP plots showing gene expression of ileal macrophage populations. J) UMAP plot of bronchoalveolar lavage macrophage populations. K) Percent of macrophages from each donor assigned to each population. L) UMAP plots showing gene expression for bronchoalveolar lavage macrophage populations. The paired t-test was used for two-way statistical comparisons, and the one-way ANOVA with Dunnett’s multiple comparisons test was used to compare mild and severe COVID-19 to healthy controls.*, p ≤ 0.05; **, p ≤ 0.01; ****, p < 0.0001; Res, Resident macrophage cluster; Inf, Inflammatory macrophage cluster; HC, healthy control; M, mild COVID-19; S, severe COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
SLAMF7 high in inflamed synovial tissue
Comparing osteoarthritis (OA) with rheumatoid arthritis, the researchers found that RA tissue contains macrophages with an inflammation signature, including the upregulation of genes that are induced by interferon as well as genes that encode inflammatory chemicals in the body. The gene with the highest increase in expression among these was SLAMF7.
This was found in low levels in joint synovial tissue from joints inflamed by OA, but at high levels in RA patients. It was present in 55% of macrophages in the latter, but <6% of patients with OA. It was present at twofold higher levels in synovial fluid macrophages from the RA group compared to the OA group, and on about half and a quarter of the macrophages in each group, respectively. However, this was not true of another SLAM receptor, pointing to SLAMF7 as being specific to inflammatory macrophages in RA.
IFN-γ mainly drives SLAMF7 overexpression
IFN-γ was found to be the primary inducer of this receptor on macrophages, along with IFN-β, IL-1β and TNF-α at lower levels. However, the pro-inflammatory cytokine IL-6 failed to induce SLAMF7 expression. Again, the former cytokines reduced the levels of another SLAM receptor, CD84, to half.
The researchers also confirmed that the JAK pathway played an important role in SLAMF7-mediated macrophage activation. The JAK inhibitor ruxolitinib suppressed it effectively. In fact, this drug is used to treat myelofibrosis, where, again, activated SLAMF7-expressing macrophages are found. It also doubles CD84 levels, which may mean that IFN-γ has opposing and reciprocal effects on these two SLAM receptors.
SLAMF7 binding triggers inflammatory cascade
In the next step, SLAMF7 expression was induced at high levels on macrophages, by IFN-γ. Subsequently, activating monoclonal antibody or recombinant SLAMF7 protein was added to bind to the receptors. The researchers found that this binding event triggered dramatic changes in gene expression.
Almost 600 genes were upregulated – the Macrophage SLAMF7 Stimulation Signature – along with an increase in several inflammatory cytokines and chemokines, even beyond the rise caused by IFN-γ alone. For instance, TNF-α and IL-6 levels increase from picomolar to nanomolar levels at this time. Moreover, the expression of SLAMF7 itself increased still further, suggesting a positive feedback loop.
Binding of macrophages to SLAMF7 also sets a myeloid inflammatory cycle into motion, besides priming the inflammasome to respond strongly to microbial TLR-binding antigens, or to cytokines, with a massive release of IL-1β. On the other hand, when a combination of IFN-γ + LPS (a potent bacterial molecule) was used to activate the macrophages, the cytokine profile characteristic of SLAMF7 binding was not observed. However, there was a partial similarity between the cytokine response and gene expression profile that occurred when IFN-γ was first used, followed by LPS exposure.
The researchers said, “This SLAMF7 activation program rests upon and is a separate step after primary stimulation of macrophages by IFN-γ or other M1 differentiation and activation factors.” The characteristic condition of this activation program - the super-activated macrophage inflammatory state induced by SLAMF7 engagement (SAM7) – is an initial potentiation of the macrophages by IFN-γ, whereby SLAMF7 is dramatically upregulated. Binding events at this receptor now complete the activation of the primed macrophages to produce a hyper-inflammatory state of macrophage activation.
Autocrine feedback loop via TNF-α
This pathway appears to be sustained and further amplified by an autocrine feedback loop, involving TNF-α, which is rapidly induced (within two hours) following SLAMF7 engagement and then continues to build up over time. Evidence for this includes the halving of TNF-α expression by anti-TNF antibody, or by silencing TNF receptors by siRNA.
SLAMF7 is a super-activator of macrophages
They found that the mean SLAMF7 activation score in RA was almost double that of individuals with OA, indicating that this receptor is closely involved in inflammation in those with RA.
Similarly, overlapping macrophage activation gene profiles were generated both in inflammatory bowel disease (IBD) and in COVID-19, compared to RA. In fact, bronchoalveolar lavage fluid cells and cells from inflamed intestinal tissue in COVID-19 and IBD showed the same macrophage activation signatures. They also noted a twofold SLAMF7 activation score in inflamed gut relative to normal gut tissue. And in severe COVID-19, the score was six times higher compared to mild COVID-19. All this suggests the dominance of the SAM7 program of macrophage activation in inflammatory conditions, especially since the SLAMF7 receptor is not found on resident macrophages in normal tissue.
Exploring different macrophage subsets, they found that inflammatory macrophages had extremely high activation scores compared to resident macrophages. Again, three distinct subsets of macrophages in COVID-19 patients were strikingly increased in proportion, with two of these groups showing expansion in severe disease. Interestingly, these had the highest activation scores, and macrophages from severely ill COVID-19 patients had extremely high SLAMF7 expression levels, along with very high levels of many other inflammatory and interferon-inducible markers and receptors.
Implications
In short, the SAM7 state may be a major component of the hyperinflammatory response seen in COVID-19 pneumonia, as well as in RA, and IBD. However, the sequential manner in which such activation is achieved also allows for the use of multiple different therapeutic countermeasures. These include JAK inhibitors like ruxolitinib, TNF-α inhibitors, or SLAMF7 blockade by Elotuzumab. The last category is especially helpful in that it may allow the SAM7 program to be inhibited selectively while still allowing macrophages to execute their normal essential immune functions.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources