Even as the coronavirus disease 2019 (COVID-19) pandemic continues to threaten a second wave of SARS-CoV-2 infection, no vaccine has been approved in phase III trials so far. Thus, the search for effective antivirals is still ongoing.
A new study describes two promising small molecules that inhibit a host cell factor, called nucleotide-binding oligomerization domain-containing protein 2 (NOD2), that inhibits the replication and spread of many arboviruses. This could lead to the development of broad-spectrum antivirals. The study's findings have been published on the preprint server bioRxiv*.
Many highly effective direct-acting antivirals have been identified for many viruses such as HIV, herpes viruses, and hepatitis C viruses. However, these tend to act specifically against one virus or closely related virus families. The current study is aimed at finding broad-spectrum antivirals that can inhibit a range of different viruses.
The researchers looked at a set of small molecules that specifically inhibit the pattern recognition receptor NOD2, as well as a molecule called RIPK2 (receptor-interacting serine/threonine-protein kinase 2) that is essential for NOD2 signaling. NOD2 receptors have bacterial cell wall ligands called muramyl dipeptide (MDP), which is a peptidoglycan, but they also bind with viral RNA.
Two faces of NOD2
NOD2 is expressed at higher levels in human fetal brain cells following Zika virus infection and stimulates this viral replication. The viral RNA-NOD2 complex causes the formation of a nodosome and promotes innate immunity.
NOD2 is a double-edged weapon, inducing primary innate immunity against several viruses but also triggering myocarditis as a result of coxsackievirus B3 infection.
Targeting NOD2 signaling
The researchers tested the impact of two drugs, GSK717, which inhibits NOD2, and GSK583, which inhibits the downstream protein, RIPK2. These were originally developed to treat inflammatory conditions.
Examining their effect on multiple human primary cell lines and tissue explants, they concluded that these molecules prevent the replication of SARS-CoV-2, flaviviruses, and alphaviruses. This ability to inhibit a range of viruses is partly due to their capacity to enhance innate immunity.
Zika-Induced nodosomes stimulate viral replication
Earlier studies have shown that Zika viruses replicate in human fetal brain cells and persist in the human fetal brain. Later, it was found that this causes an increased expression of many inflammasome genes, including NOD2, which was increased by over a hundredfold. Other inflammasome stimulators include viral double-stranded RNA and the human recombinant interferon-alpha.
NOD2 upregulation was key to Zika virus replication. This was found to operate via NOD2-induced downregulation of several interferon-stimulated genes (ISGs) and inflammasome-related genes, which indicates inhibition of the innate immune response.
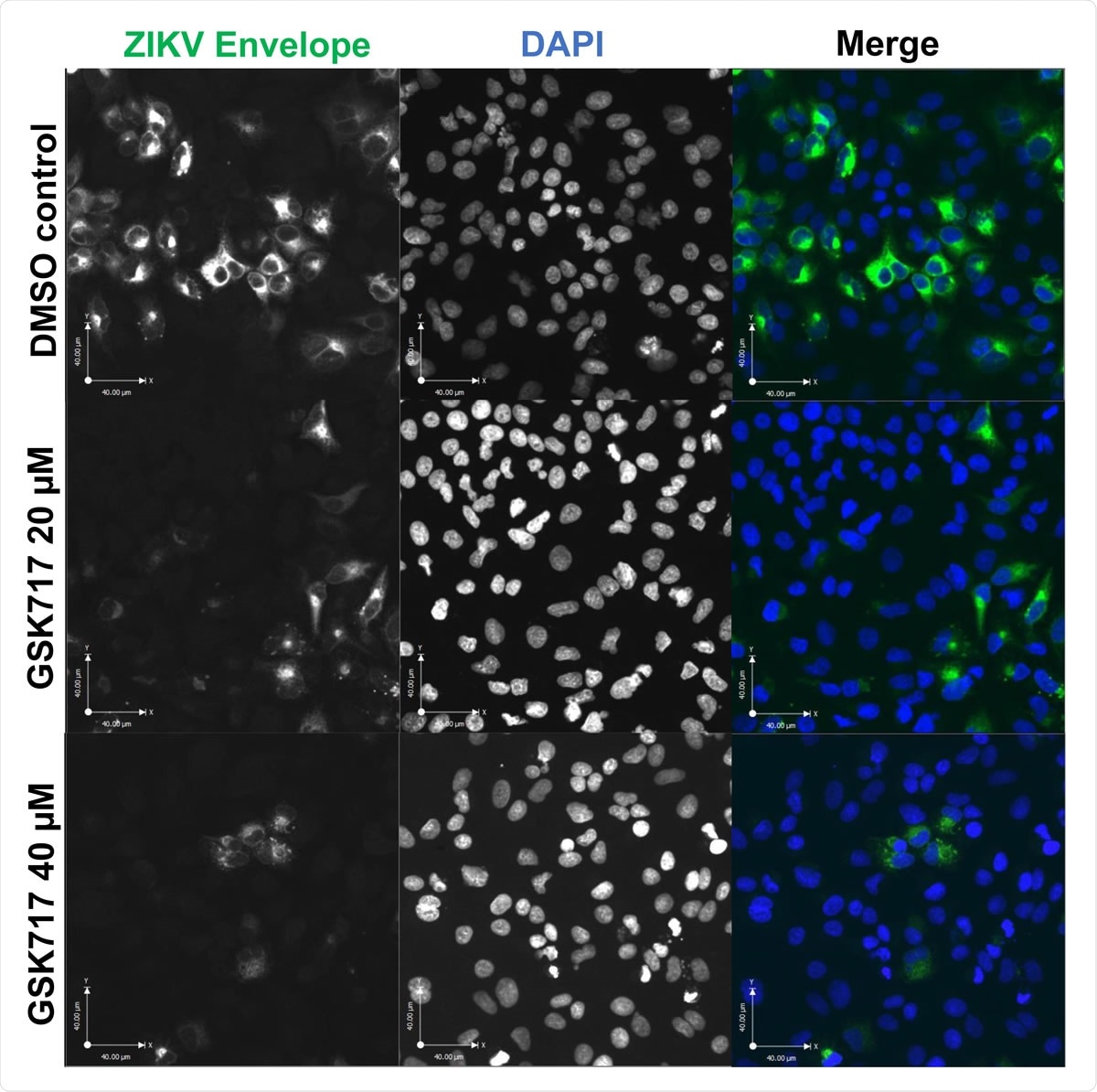
The anti-NOD2 drug GSK717 blocks the spread of ZIKV infection. (A) Representative confocal imaging (20X) showing antiviral effect of GSK717 at 20 µM and 40 µM. A549 cells were infected with ZIKV (MOI=1) followed by treatment with DMSO or GSK717 at 20 or 40 µM for 48 hours before processing for indirect immunofluorescence. ZIKV-infected cells were identified using a mouse monoclonal antibody (4G2) to envelope protein and Alexa Fluor 488 donkey anti-mouse to detect the primary antibody. Nuclei were stained with DAPI. Images were acquired using a spinning disk confocal microscope equipped with Volocity 6.2.1 software.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
NOD2 inhibition prevents Zika replication
When NOD2 expression was silenced by the tested inhibitors GSK717 and GSK583, no cytotoxicity effect was observed, but the multiplication and spread of the virus were inhibited. This was seen not only in fetal brain tissue but also in human primary embryonic lung fibroblasts.
The researchers found that this anti-Zika effect was present in several cell lines, including those derived from the lung and hepatoma cells, and that GSK717 acted in a dose-dependent fashion, whether at high or low infectious doses of the virus.
NOD2 inhibition prevents dengue/MAYV replication
They also tested its ability to suppress the replication of the dengue virus, "the most important arbovirus in terms of morbidity and mortality and the causative agent of 129 Dengue Hemorrhagic Fever/Dengue Shock Syndrome." The GSK717-treated cells were found to have reduced viral titers by over 90% compared to untreated cells when treated post-infection, and a steep decline in the number of cells expressing the dengue virus antigen was also observed after incubating with the drug for 48 hours. This held good, with a 60% reduction in Zika and Dengue virus genomic RNA levels in coinfected cells treated with GSK717.
GSK717 also reduced the titer of mosquito-transmitted alphavirus (MAYV), both alone and in cells coinfected with this virus and dengue virus – with a greater impact on the former virus. SARS-CoV-2 titers were also reduced to a similar extent by NOD2 inhibition.
RIPK2 inhibition suppresses arbovirus replication
The NOD2-RIPK2 interaction triggered by the binding of MDP to NOD2 causes transcription of several inflammatory factors and anti-bacterial proteins. However, Zika-infected cells did not show higher levels of RIPK2 transcription.
The researchers, therefore, asked if inhibition of RIPK2-NOD2 binding would affect the replication of arboviruses such as dengue, Zika and MAYV. This was found to be the case, at both 12 and 24 hours after infection, in cells treated with the RIPK2 inhibitor GSK583.
Successful SARS-CoV-2 inhibition in vitro
This viral suppressive effect was also observed with SARS-CoV-2, whether the drug was used before or after viral infection occurred. The observed reduction in SARS-CoV-2 titer was to the tune of 60% and 90%, respectively, at the highest drug concentration, in two separate human lung and liver tumor-derived cell lines, but no evidence of cytotoxicity was found.
Implications
The arboviruses tested in this study are found to circulate in the same regions of the world, and often cause similar clinical signs and symptoms. Serological cross-reactivity further complicates the diagnosis. Thus, a drug capable of targeting all these viruses would be a boon indeed.
At the same time, the task of developing an effective drug to tackle the COVID-19 pandemic is becoming more urgent by the day. Thus, the robust antiviral activity shown by both GSK717 and GSK583 against flaviviruses and against SARS-CoV-2 as well holds great promise for their further development.
Gefitinib is a monoclonal antibody that targets the growth factor receptor EGFR as well as RIPK2. Its inhibitory effect against dengue virus replication and inflammatory cytokine release has been documented. The authors suggest that it may well be useful in dengue patients, and similarly, NOD2 and RIPK2 inhibitors may turn out to be effective anti-COVID-19 (as well as anti-flaviviral) drugs.
Both these drugs were actually developed to target inflammatory immunological diseases. Therefore, an additional advantage of these drugs is their anti-inflammatory effect. Since the viral conditions caused by alpha-, flavi- and SARS-CoV-2 owe their severity mostly to the associated hyper-inflammatory phenomena, these drugs could mitigate these clinical syndromes.
And lastly, several vaccine adjuvants enhance NOD2, in order to improve the immune response. In the light of this study, however, this action could be undesirable since higher NOD2 expression increases the replication of several RNA pathogenic viruses. In fact, this may be one of the viral evasion mechanisms by which the virus successfully prevents host immune responses.
The researchers conclude, "The current study illustrates how the identification of a drug target through transcriptomic analyses of virus-infected cells can lead to novel broad-acting host-directed antiviral strategies with a high barrier of resistance."
Using this mode of research, they were able to find broad-spectrum antivirals among nodosome inhibitors, which may well repay further attention as potentially effective and safe anti-COVID-19 drugs.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Limonta, D. et al, (2020). Nodosome Inhibition as A Novel Broad-Spectrum Antiviral Strategy Against Arboviruses And SARS-CoV-2. bioRxiv preprint. doi: https://doi.org/10.1101/2020.11.05.370767, https://www.biorxiv.org/content/10.1101/2020.11.05.370767v2
- Peer reviewed and published scientific report.
Limonta, Daniel, Lovely Dyna-Dagman, William Branton, Valeria Mancinelli, Tadashi Makio, Richard W. Wozniak, Christopher Power, and Tom C. Hobman. 2021. “Nodosome Inhibition as a Novel Broad-Spectrum Antiviral Strategy against Arboviruses, Enteroviruses, and SARS-CoV-2.” Antimicrobial Agents and Chemotherapy 65 (8). https://doi.org/10.1128/aac.00491-21. https://journals.asm.org/doi/10.1128/AAC.00491-21.