Loss of smell and taste are among the most specific early warning symptoms of infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the pathogen that causes coronavirus disease 2019 (COVID-19). Many theories have arisen regarding the origin of this clinical symptom.
How smell occurs
High in the nasal cavity are olfactory receptors (ORs). ORs are the terminals of the olfactory sensory neurons (OSNs) located in the olfactory epithelium. When an odor-causing substance binds to the ORs, the first step of smelling has taken place. The OSN axons then travel deeper to contact the glomerulus of the olfactory bulb (OB) in the nose. The OSN also forms synapses with second-order neurons, which carry the odorant-OR binding-induced impulse into the olfactory cortex, the part of the brain that processes odor perception.
Olfactory dysfunction, resulting in the loss or alterations of the sense of smell, has been reported as a characteristic feature of many COVID-19 infections.
The human nose expresses the SARS-CoV-2 entry receptor, the angiotensin-converting enzyme 2 (ACE2), at high levels on both the respiratory and olfactory parts of its inner epithelial lining.
A previous hamster study showed many olfactory epithelium (OE) cells to be infected by this virus. The OB contains vascular pericytes that express ACE2 at high levels. These cells play a major role in keeping the blood-brain barrier intact and also regulate host immunity and blood pressure. The OB is attacked by respiratory viruses such as the flu virus and the respiratory syncytial virus (RSV).
SARS-CoV-2 infects OE
The current study used a humanized ACE2-expressing mouse model to understand how the virus affects the OE. The researchers infected 6-8-week-old mice with the virus. At 2 and 4 days post-infection (dpi), they found that the nasal respiratory epithelium (RE) and the airway tissue up to the level of the lungs were acutely infected, with the peak viral load at 2 dpi. The viral nucleocapsid (N) protein was also abundant in the lung tissue.
They found that the viral RNA load was present at high levels in the olfactory mucosa at 2 dpi, persisting through to 4 dpi. However, it was lower in the OB and other brain regions. The same pattern was found, with the N protein being detected in abundance in the OE but not in the OB.
Olfactory testing in the infected mice showed that they took much longer to detect food pellets, almost double the time taken by controls. In 2 out of 13 mice, anosmia was present. At 4 dpi, however, the mice recovered from their anosmia, with infected and control mice showing the same ability to detect food pellets. Thus, this showed that the virus infects the OE in mice, causing olfactory dysfunction.
SARS-CoV-2 attacks non-nerve cells in OE
The olfactory membrane is made up of both the OE and the lamina propria, a connective tissue layer underneath. The OE comprises a variety of cells, both olfactory and supporting cells. ACE2 expression was found to be mostly in the supporting cells, rather than the neuroepithelial cells.
The same results were found on immunoassay using the anti-SARS-CoV-2 N protein, with the non-neuroepithelial cells showing a high level of N protein expression. In short, the sustentacular and Bowman’s gland cells were the major viral targets, with microvillar and OSN progenitors being affected to a much smaller extent at 4 dpi. Even then, the latter was adjacent to infected supporting cells.
The results suggest that the primary viral target is the supporting cells of the OE, spreading later on into the neuroepithelial cells.
SARS-CoV-2 causes OE death and immune cell infiltration
Following infection with the virus, the OE lost its structural organization, with severe damage to the layer of cilia originating in the OSNs and the microvilli of the supporting cells. Severe apoptosis of the OE was observed, in the supporting cells, and the nerve bundles originating from the neuroepithelial cells.
Immune cells, including macrophages and dendritic cells, were also seen to infiltrate the OE in infected mice. This included an influx of cytotoxic CD8 T cells, which release perforin and granzyme B at high levels. Such destructive enzymes would further damage the epithelial integrity. All these changes are potential reasons for the loss of smell following SARS-CoV-2 infection.
Regeneration of OE
Whereas the immature olfactory neuroepithelial cells are in a dormant state before infection, they switch into the active state and begin to grow upwards from the basal layer of the OE into the upper layer. This indicates a regeneration of the damaged OE, mediated by the proliferation of olfactory stem cells, which differentiate into olfactory neurons and their supporting cells. This is responsible for the return of smell.
SARS-CoV-2 infection induces inflammatory response
The OE showed a change in the expression level of hundreds of genes, because of the hijacking of the cellular machinery via viral replication. Many of these genes induce antiviral and inflammatory pathways in the OE at 2 dpi, but their expression dropped at 4 dpi. Other upregulated genes involved apoptosis of cells and regulation of neuronal projection. This antiviral response was not seen in the OB.
Many of the downregulated genes related to the transduction of olfactory impulses, including ORs, may also explain the olfactory dysfunction.
Restriction and restoration of damaged OE
The robust viral replication and antiviral response found in the OE but not the OB suggests that the virus is restricted to the OM and does not spread to other parts of the brain. One reason for this could be the interferon-dependent pathways that are activated by the infection since this forms an effective barrier against viral invasion to the brain. Again, the apoptosis of OSNs following their infection could limit the spread of the virus into the CNS when the OE undergoes destruction.
Damage to the supporting cells of the OE, along with the inflammatory infiltration triggered by the infection, contributes to the severe disorganization of the OE and results in anosmia. The question remains, however, as to how and why the viral RNA was found in the mature OSNs and immature progenitors following infection, despite their lack of ACE2 expression. This may signal that receptors other than ACE2 are involved in viral entry and spread.
Many ORs showed reduced expression after infection, perhaps because of the type I interferon pathway induced by the virus in the OE. This response is linked to a decreased sense of smell and lower OE expression, as shown by a recent study.
The current study also showed that the Rtp1 was also downregulated in this infection. This protein increases the expression of ORs at the cell surface. Thus, its downregulation could also contribute to anosmia.
The inflammatory response also promotes stem cell differentiation and OE regeneration. Thus, the observed restoration of olfactory function is also explained by these findings.
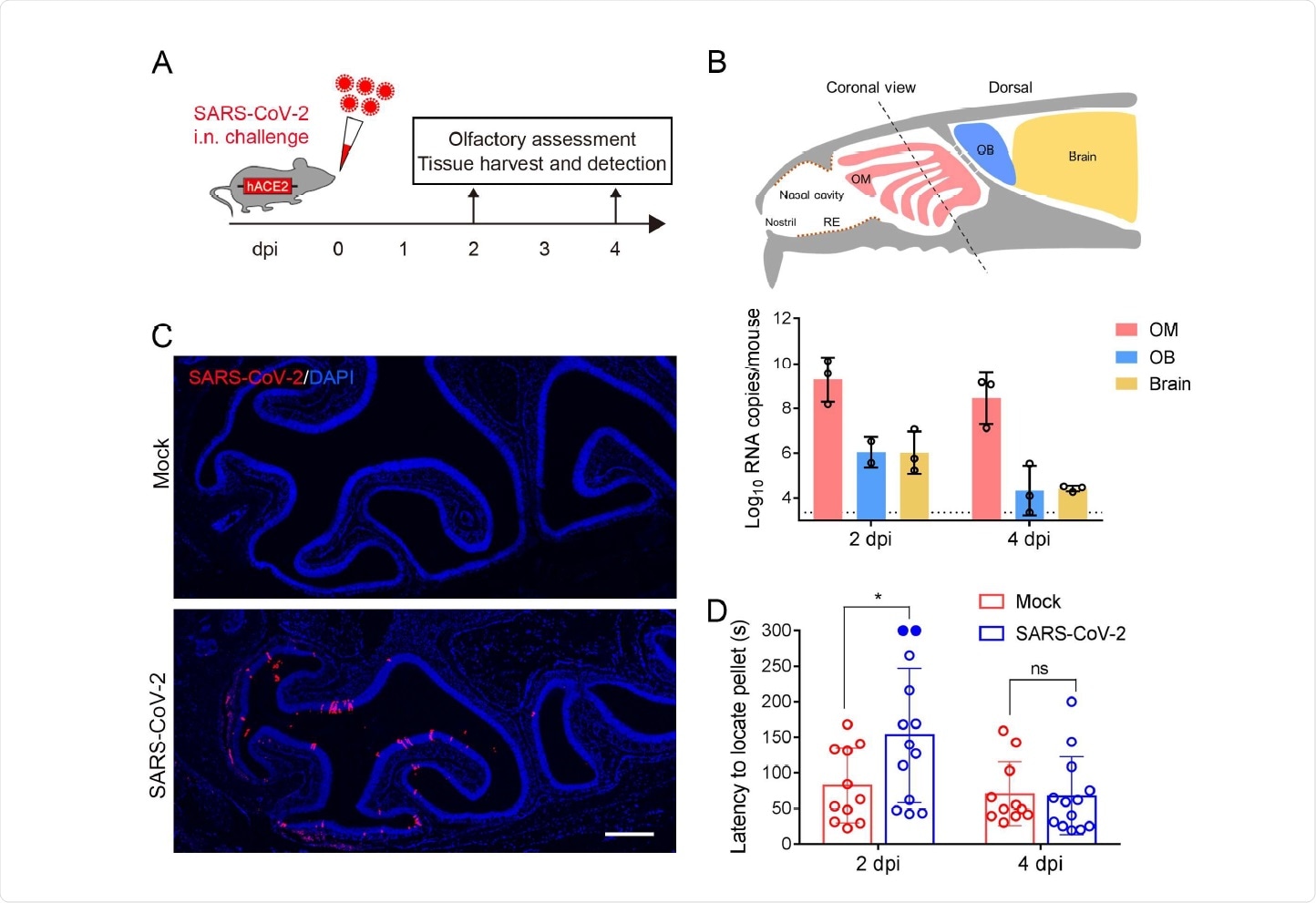
SARS-CoV-2 primarily infects the OE and causes olfactory dysfunction in hACE2 mice. (A) Schematic diagram of experimental design. Briefly, groups of 6-8 weeks old hACE2 mice were infected with 5.4 × 105 PFU of SARS-CoV-2 intranasally. Olfactory function of infected mice was measured by the buried food pellet test at indicated times post inoculation. Mice were sacrificed at 2 dpi and 4 dpi for viral detection and histopathological analysis. (B) Schematic view of the OM in the nasal cavity of mice in a sagittal plane, the dotted line indicated a coronal section (upper). And viral RNA copies were determined by real time qPCR and shown as mean ± SD from three independent replicates (lower). (C) Immunostaining of OM from SARS-CoV-2 infected mice for SARS-CoV-2 N protein (red) and DAPI (blue). Scale bar, 400 µm. (D) Buried food pellet test. Latency to locate the food pellets for mice infected with SARS-CoV-2 (n=13) or DMEM (n=11) was measured at 2 dpi and 4 dpi.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Implications
The authors say, “Our study established a mouse model of olfactory dysfunction induced by SARS-CoV-2. The animal model of olfactory disorders is available to subsequently evaluate the antiviral drugs as well as vaccines for the inhibition of SARS-CoV-2 and the improvement of post-viral olfactory disorders.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Qin, C.-F., et al. (2020). SARS-CoV-2 infection causes transient olfactory dysfunction in mice. bioRxiv preprint. doi: https://doi.org/10.1101/2020.11.10.376673, https://www.biorxiv.org/content/10.1101/2020.11.10.376673v1
- Peer reviewed and published scientific report.
Ye, Qing, Jia Zhou, Qi He, Rui-Ting Li, Guan Yang, Yao Zhang, Shu-Jia Wu, et al. 2021. “SARS-CoV-2 Infection in the Mouse Olfactory System.” Cell Discovery 7 (1). https://doi.org/10.1038/s41421-021-00290-1. https://www.nature.com/articles/s41421-021-00290-1.