Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus, originated from Wuhan, China, in late December 2019 and rapidly spread across the globe, progressing into a devastating pandemic. To date, the COVID-19 pandemic has caused 54.3 million infections and over 1.31 million deaths worldwide.
Coronavirus disease (COVID-19) affects the upper and lower respiratory systems and causes flu-like symptoms in most infected people. Although many people infected by SARS-CoV-2 experience mild symptoms, some patients have severe symptoms leading to massive lung damage. Treatment options for COVID-19 are limited, and mortality rates range from 0.5% to 5%.
The Johns Hopkins COVID Resource Center estimates more than 11 million infections and over 246,000 deaths due to COVID-19 in the United States as of November 15, 2020. Although a preventive vaccine for SARS-CoV-2 could eventually become available, unless sufficient herd immunity is achieved, COVID-19 could potentially cause significant morbidity and mortality for the next couple of years.
In the absence of a protective vaccine, COVID-19 has been tackled using non-pharmaceutical public health interventions such as lockdowns, social isolation, quarantine, and use of personal protective equipment. Clearly, a protective vaccine against SARS-CoV-2 and effective therapeutics for COVID-19 disease are needed urgently to fight this unprecedented pandemic.
Evaluating the in vitro activity of stenoparib against SARS-CoV-2
A team of researchers from the Northern Arizona University evaluated a new PARP inhibitor, stenoparib, which has recently advanced to Stage II of clinical trials to treat ovarian cancer. Their study, an initial report on in vitro stenoparib activity against human coronaviruses such as SARS-CoV-2, has been published on the preprint server bioRxiv*.
During the study, stenoparib exhibited dose-dependent suppression of multiplication and spread of SARS-CoV-2 in Vero E6 monkey kidney and Calu-3 human lung adenocarcinoma cells. Stenoparib also strongly inhibited the multiplication of the seasonal HCoV NL63 human respiratory coronavirus.
Stenoparib blocks both virus entry and post-entry processes
Time-of-addition experiments show that stenoparib inhibits both the entry of virus and post-entry events compared to the drug remdesivir, which inhibits the replication of virus post entry into the host cell. In addition, a 10 µM dose of stenoparib combined with 0.5 µM remdesivir suppressed the growth of coronavirus by 90.7%, which indicates a strong synergistic effect for this combination of drugs. It is interesting to note that a 10 µM dose of stenoparib is far below its 25.5 µM half-maximally effective concentration (EC50).
“Overall, our observations imply multiple mechanisms for stenoparib, including impeding of viral entry and intracellular growth via modifications of multiple viral and host proteins.”
Based on these results, the authors concluded that stenoparib can potentially be a valuable weapon in the fight against the COVID-19 pandemic, either as a standalone solution or in combination with drugs like remdesivir.
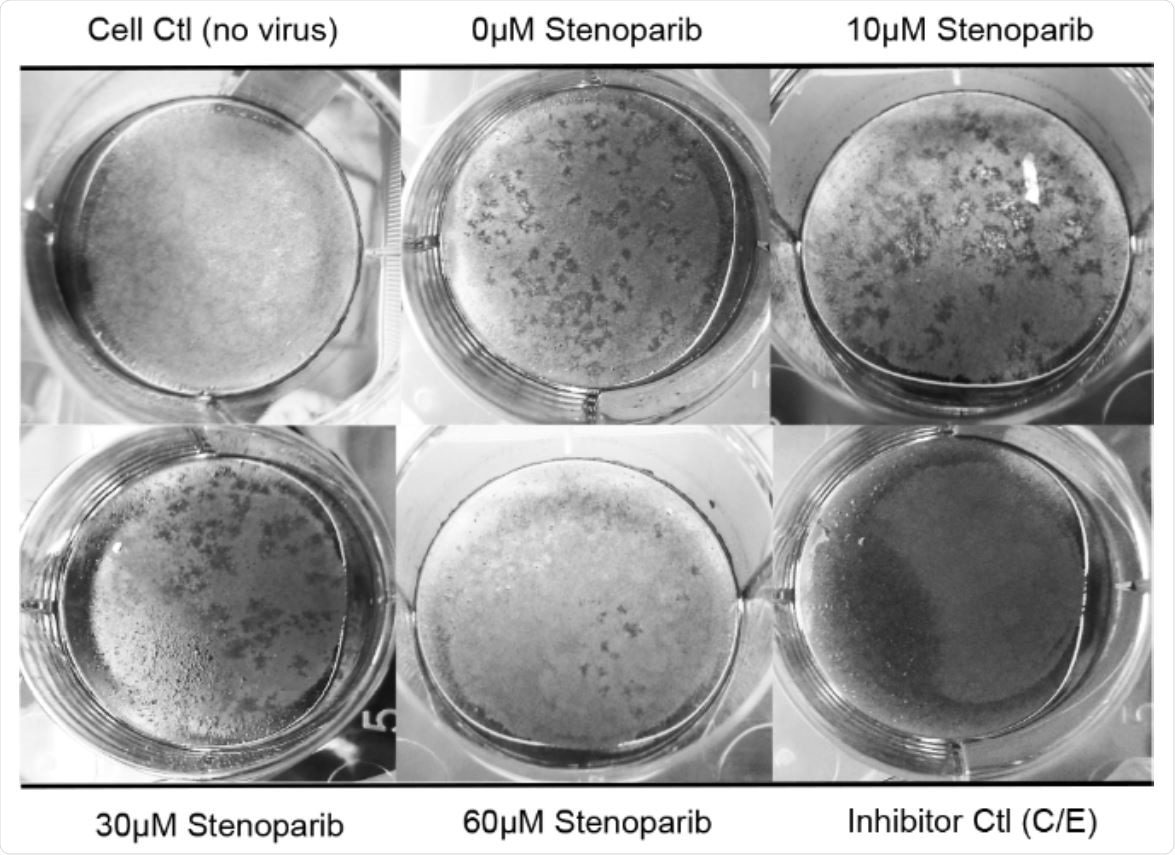
Stenoparib inhibits plaque formation in Calu-3 cells. Plaque assays were performed using Calu-3 cells infected with SARS-CoV-2 and treated with varying concentrations of stenoparib. Plaques are identified as empty regions or “dead zones” in the cellular monolayer and are expressed as plaque forming units (PFUs) per well. Plaques were manually counted and averaged among experimental replicates. This score is normalized as a percentage of untreated, but infected cells. In this representative image of the SARS-CoV-2/Calu-3 experiment, plaques are dark scars on the cellular monolayer. Controls were 1) uninfected cells (Cell Ctl), 2) untreated, but infected cells (0 μM), and 3) a camostat mesylate and E64d (C/E) inhibitor control (Inhibitor Ctl). Treatment with stenoparib at 10 μM and 30 μM led to a marked reduction in plaquing efficiency, whereas 60 μM resulted in near complete inhibition.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Stenoparib’s broad range of action may offer a therapeutic advantage over current options
In light of the unprecedented threat to global public health and economies, it is clear that there is an urgent need for new therapeutics in the fight against COVID-19. Repurposing existing drugs that are either approved for human use or in advanced stages of the clinical trials can significantly help advance toward this goal.
The researchers believe that stenoparib, the PARP inhibitor that is currently in Stage II clinical trials for ovarian cancer treatment, might be such a drug, as the safety and dosage of stenoparib in humans has already been established. The results of this study indicate that stenoparib has potent, in vitro antiviral activity against the SARS-CoV-2 virus and other coronaviruses. This antiviral activity seems to be based on multiple modes of action, which inhibit both pre-and post-entry viral replication events.
The authors state that this broad range of action of stenoparib may offer a therapeutic advantage over other options currently available that have a narrower mode of action. Moreover, their results clearly suggest that a combination of stenoparib and remdesivir may be a potent therapeutic solution against coronavirus infection.
“Considering their distinct mechanisms and high potency, a combination of remdesivir and stenoparib is likely to produce a synergistic effect on additional SARS-family coronaviruses, including SARS-CoV-2.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Stenoparib, an inhibitor of cellular poly (ADP-ribose) polymerase (PARP), blocks replication of the SARS-CoV-2 human coronavirus in vitro Nathan E. Stone, Sierra A. Jaramillo, Ashley N. Jones, Adam J. Vazquez, Madison Martz, Lora M. Versluis, Marlee O. Raniere, Haley E. Nunnally, Katherine E. Zarn, Roxanne Nottingham, Jason W. Sahl, David M. Wagner, Steen Knudsen, Erik W. Settles, Paul S. Keim, Christopher T. French bioRxiv 2020.11.12.380394; doi: https://doi.org/10.1101/2020.11.12.380394, https://www.biorxiv.org/content/10.1101/2020.11.12.380394v1
- Peer reviewed and published scientific report.
Stone, Nathan E., Sierra A. Jaramillo, Ashley N. Jones, Adam J. Vazquez, Madison Martz, Lora M. Versluis, Marlee O. Raniere, et al. 2021. “Stenoparib, an Inhibitor of Cellular Poly(ADP-Ribose) Polymerase, Blocks Replication of the SARS-CoV-2 and HCoV-NL63 Human Coronaviruses in Vitro.” Edited by Diane E. Griffin. MBio 12 (1). https://doi.org/10.1128/mbio.03495-20. https://journals.asm.org/doi/10.1128/mBio.03495-20.