With the coronavirus disease 2019 (COVID-19) pandemic infecting over 52.7 million people around the world, and killing over 1.2 million, a large amount of research has tried to better understand immune responses against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the pathogen that causes COVID-19.
A research team from the Howard Hughes Medical Institute, USA, has studied the evolution of the antibodies that fight against SARS-CoV-2 infection. Their study titled, “Evolution of Antibody Immunity to SARS-CoV-2,” was released on the preprint server bioRxiv*.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
SARS-CoV-2 infection triggers the development of antibodies that can neutralize the antigens of the virus – notably the spike protein that the virus uses to enter host cells. They thus protect an individual from re-infection. The levels of antibodies developed against the infection may vary among persons, and with time the antibody levels are also found to decrease. However, the B cells of the immune system have a specific type called memory B cells. These memory B cells can be “called upon to produce antibodies upon re-infection,” suggest the researchers. This type of immune response is called humoral memory response.
This use of memory B cells and their robustness in the prevention of re-infection has not yet been explored, the team explains.
Study design
For this study, the team of researchers looked at the humoral memory response among 87 persons. The participants’ humoral antibody response was studied at 1.3 months and 6.2 months after their infection with SARS-CoV-2. Nussenzweig, an immunologist at The Rockefeller University in New York City and contributor to the research project, explains: “Our idea was that if we’re able to find such neutralizing antibodies, we would know what part of the virus vaccines have to target.”
Findings
Overall, results of the study were:
Antibodies decline over time
- Antibody responses to SARS-CoV-2 were found at around 1.3 months or 40 days after the infection
- The IgM, IgG and IgA anti-RBD (receptor-binding domain) antibodies in plasma decreased between 1.3 and 6.2 months after recovery from infection
- Antibody type IgM showed the greatest decrease in anti-RBD reactivity (53 percent), followed by IgG (33 percent), while IgA decreased by only 15 percent
- Those patients who had prolonged symptoms had significantly higher anti-RBD IgG and anti-N antibody levels at both time points
- Although antibodies to RBD and plasma neutralizing activity decrease significantly, they were still detectable 6.2 months after infection in most patients
Memory B cells
- Plasma antibody levels fell over time, but the memory B cells were found to contribute to immune response late after the recovery
- For this, the team used flow cytometry to isolate the B cells that contained receptors that bound to the RBD.
- Memory B cells that bound to RBD rose significantly between 1.3 and 6.2 months
- Authors wrote, “We conclude that while the magnitude of the RBD-specific memory B cell compartment is conserved between 1.3 and 6.2 months after SARS-CoV-2 infection, there is significant clonal turnover and antibody sequence evolution...”
Evolution of antibodies
- Antibody evolution occurs due to mutation at the germinal centers. Here the antigen from the virus is retained in the form of immune complexes. These complexes of antigens and antibodies lie on the surface of follicular dendritic cells for a long time
- The viruses that remain in the body after recovery act as a source of the viral antigens for the antibodies to develop. They may be present in the gut, the team found.
- Electron tomography and other methods helped in finding these residual viruses
- Mutations were low in antibodies generated early after the infection recovery, the team noted.
- The team writes, “anti-SARS-CoV-2 memory B cell response evolves during the first 6 months after infection...”
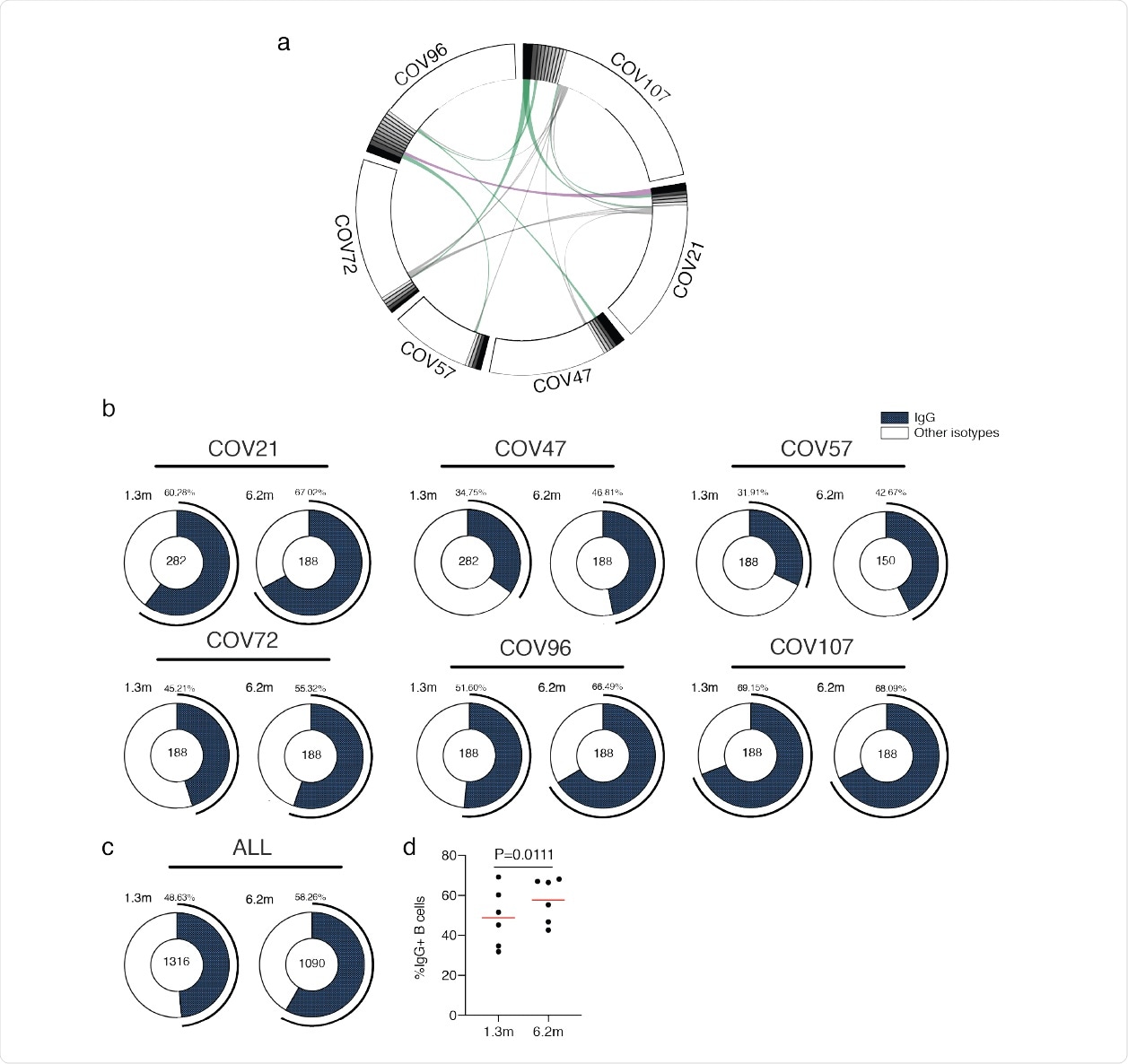
Circos plots and IgG positive RBD specific B cells a, Sequences from all six individuals with clonal relationships depicted. Interconnecting lines indicate the relationship between antibodies that share V and J gene segment sequences at both IGH and IGL. Purple, green and grey lines connect related clones, clones and singles, and singles to each other, respectively. b, For each patient, the number of IgG heavy chain sequences (black) analyzed from six individuals at month 694 1.3 (left panel) or month 6.2 post infection (right panel). The number in the inner circle indicates the number of cells that was sorted for each individual denoted above the circle. c, The same as b but showing the all 6 patients combined data. d, The comparison of the percentage of IgG positive B cells from six individuals at month 1.3 or month 6.2 post-infection. The horizontal bars indicate the mean. Statistical significance was determined using paired t test.
Conclusions and implications
The researchers found, “Memory responses are responsible for protection from re-infection and are essential for effective vaccination.” This evolution of the memory B cells takes place between 1.3 and 6.2 months after infection. With the persistent presence of antigens in the form of residual virus, the antibodies continue to evolve.
Nussenzweig, said in a statement, “the really good news is that people who are infected are very unlikely to become sick again for at least six months.” Christian Gaebler, another researcher on the project, said, “our results showed that it’s not hard for our immune systems to make effective antibodies to SARS-CoV-2.” He was also talking about their earlier study published in the journal Nature, in June this year (2020).
“One of the things people worry about a lot is what’s going to happen in six months or a year,” Nussenzweig said. “Are individuals who’ve recovered from COVID-19 still going to be protected?” This study shows that the answer may be a “yes,” especially for those who had a persistent presence of the virus in their bodies – particularly the gut, say the researchers.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Gaebler, Christian and Zijun Wang, Julio C. C. Lorenzi, Frauke Muecksch, Shlomo Finkin, Minami Tokuyama, Mark Ladinsky, Alice Cho, Mila Jankovic, Dennis Schaefer-Babajew, Thiago Y. Oliveira, Melissa Cipolla, Charlotte Viant, Christopher O. Barnes, Arlene Hurley, Martina Turroja, Kristie Gordon, Katrina G. Millard, Victor Ramos, Fabian Schmidt, Yiska Weisblum, Divya Jha, Michael Tankelevich, Jim Yee, Irina Shimeliovich, Davide F. Robbiani, Zhen Zhao, Anna Gazumyan, Theodora Hatziioannou, Pamela J. Bjorkman, Saurabh Mehandru, Paul D. Bieniasz, Marina Caskey, Michel C. Nussenzweig (2020) Evolution of Antibody Immunity to SARS-CoV-2. bioRxiv; doi: https://doi.org/10.1101/2020.11.03.367391, https://www.biorxiv.org/content/10.1101/2020.11.03.367391v1
- Peer reviewed and published scientific report.
Gaebler, Christian, Zijun Wang, Julio C. C. Lorenzi, Frauke Muecksch, Shlomo Finkin, Minami Tokuyama, Alice Cho, et al. 2021. “Evolution of Antibody Immunity to SARS-CoV-2.” Nature 591 (January). https://doi.org/10.1038/s41586-021-03207-w. https://www.nature.com/articles/s41586-021-03207-w.