In the face of discouraging news about the resurgence of COVID-19, some reports offer hope of protection in the face of a robust and persistent immune response. In an encouraging report, an Austrian preprint appearing on the medRxiv* server in November 2020 suggests a high level of durable immunity to SARS-CoV-2 over at least six months. This could shape future strategies for population testing and immunization.
The COVID-19 pandemic is far from losing its strength, with a second wave being reported in many countries in Europe. Even eleven months into the pandemic, it is unclear how robust the antibody responses are, how long they persist, as well as how well they protect against reinfection.
A typical antibody response begins with immunoglobulin M (IgM) secretion followed by a switch to IgG, the latter indicating a mature and durable immune response. earlier research has shown that over 90% of SARS-CoV-2 infected individuals have a strong antibody response targeting the viral spike protein. Since this protein contains the receptor-binding domain (RBD) where the virus engages the human host cell via the angiotensin-converting enzyme 2 (ACE2), antibodies directed against it neutralize the virus.
The current study is a follow-up of a seroprevalence survey carried out in June 2020 in an Austrian town called Weißenkirchen. This town was badly hit by the infection in the first wave. The researchers performed an ELISA-based survey of anti-SARS-CoV-2 spike IgG and IgM levels, finding a 12% prevalence, 9% showing IgG and 9% IgA, respectively, and 6% showing both.
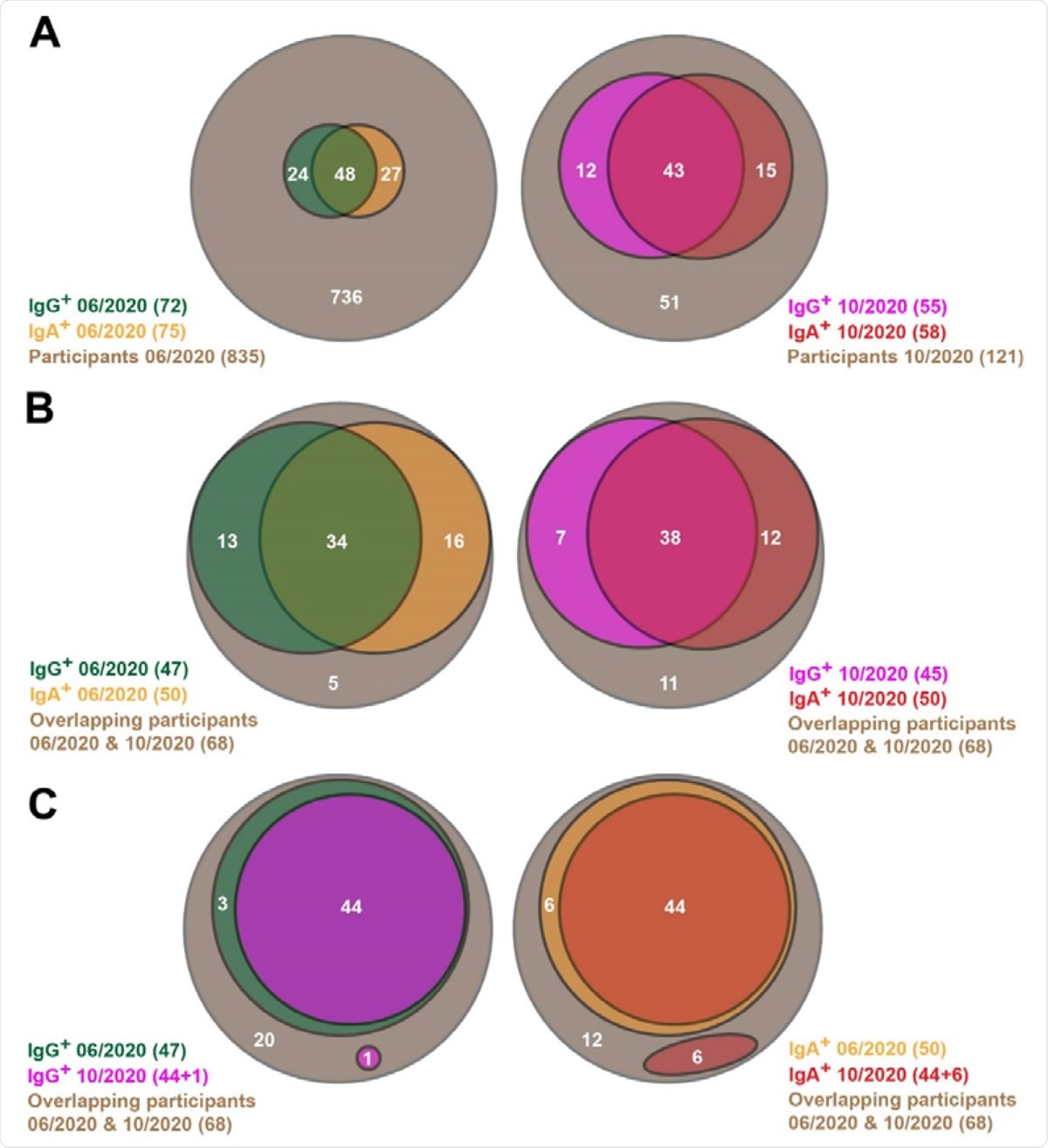
Venn diagrams showing SARS-CoV-2-specific antibody prevalence in the pilot (06/2020) and the follow-up (10/2020) studies. (A) All participants of both studies are shown here. (B, C) Only the participants are considered here, which are involved in both the pilot and follow-up studies.
The current study repeated the testing on 121 people, of whom 68 had been tested in the first wave. The researchers found that 58% (70/121) were seropositive for Immunoglobulin A (IgA) or Immunoglobulin G (IgG), with 45% and 48% having IgG and IgA antibodies, respectively. Both antibodies were present in 36%.
Among the 68 first-wave positives, 63 (93%) remained positive for anti-SARS-CoV-2 antibodies in June, with half of them having IgG and three-quarters IgA antibodies. Both antibodies were present in 56% of the group.
In the current (October) study, 84% (57/68) of this group continued to be seropositive, with 66% and 74% displaying IgG and IgA antibodies, respectively, and 56% having both. This is only slightly down from 93% in the first round of testing, which indicates that the antibodies elicited by the infection remained remarkably stable throughout this period.
To distinguish the contribution of true antibody persistence vs. a rise in antibodies caused by new infections in hitherto naïve individuals within this subgroup, the individual antibody titers were analyzed. This showed that of the 47 people who had IgG against the virus in June 2020, 44 (94%) remained positive in the current round, while 1 of the 68 had newly developed IgG. The remaining 3 did not have IgA at the beginning and lost their IgG positivity by October.
Similarly, of the 50 IgA positives in the first round, 44 (88%) had persistent IgA antibodies, but 6 others had new IgA responses. Five of these novel IgA responses were in individuals who already had IgG antibodies in June. Therefore, the researchers found, the seropositivity is primarily due to persistent antibodies. In fact, the robust IgA persistence is unexpected since this is typically part of an early and temporary immune response, with IgG being the persistent class.
Of the 34 subjects with both antibody classes, 33, that is, 97%, still had both in the second round, while the IgA level fell in one person. Thus, having both the antibodies best predicted antibody persistence in this study.
Again, to rule out undetected waning of antibody levels, the researchers compared the relative titers of both antibody classes from both rounds, using semi-quantitative ELISA. This confirmed a steady level for both classes, if not an increase, indicating a durable antibody-based immune response for six months or more. This suggests that a SARS-CoV-2 vaccine should produce both IgA and IgG antibodies and that these will provide immunity for over six months.
The authors intend to continue testing this cohort with both semi-quantitative and quantitative ELISA for both IgG and IgA and to use newer tests for T cell-mediated immunity to the virus as well. These studies will also indicate whether waning of the immune response is to be expected and the risk factors for such waning, such as patient-specific factors like age, sex and habits, disease severity, and the presence of other comorbidities.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources