A team of scientists from Japan has identified the approved antimalarial agent, mefloquine, as a potential candidate to treat severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Specifically, mefloquine has been shown to prevent the entry of SARS-CoV-2 into host cells and reduce the viral load in vitro. The study is currently available on the bioRxiv* preprint server.
The recent pandemic of coronavirus disease 2019 (COVID-19) has put a substantial amount of burden on healthcare systems and socioeconomic structures of many countries across the world.
SARS-CoV-2, the causative pathogen of COVID-19 disease, is a single-stranded, positive-sense RNA virus that rapidly spread from human to human primarily via respiratory droplets. The spike protein of SARS-CoV-2 interacts with the host cell membrane receptor angiotensin-converting enzyme 2 (ACE2) to enter and infect the host cells.
Because of the unavailability of specific therapeutics against SARS-CoV-2, in-hospital treatment of moderately or severely affected COVID-19 patients mostly rely on repurposed drugs, such as remdesivir, hydroxychloroquine, and interferon. However, further evaluation is necessary to evaluate the exact efficacy of these drugs in treating COVID-19 patients.
The application of anti-malarial drugs, such as chloroquine and hydroxychloroquine, as repurposed COVID-19 drugs, has been initiated during the early phase of the pandemic. However, many subsequent clinical trials conducted have failed to prove the expected efficacy of these drugs in COVID-19 patients. As a result, the US Food and Drug Administration (FDA) has canceled the emergency use of these drugs for COVID-19 treatment in June 2020. Given the ever-growing COVID-19 trajectories, it is of prime importance to identify new therapeutics with potent anti-SARS-CoV-2 activity.
Current study design
The scientists conducted a cell-based functional screening of many anti-parasitic/anti-protozoal drugs to identify new drug candidates that may offer anti-SARS-CoV-2 activity. To check the efficacy of the selected candidate drug, they conducted a series of in vitro experiments using human lung epithelial cells and TMPRSS2-overexpressing VeroE6 cells.
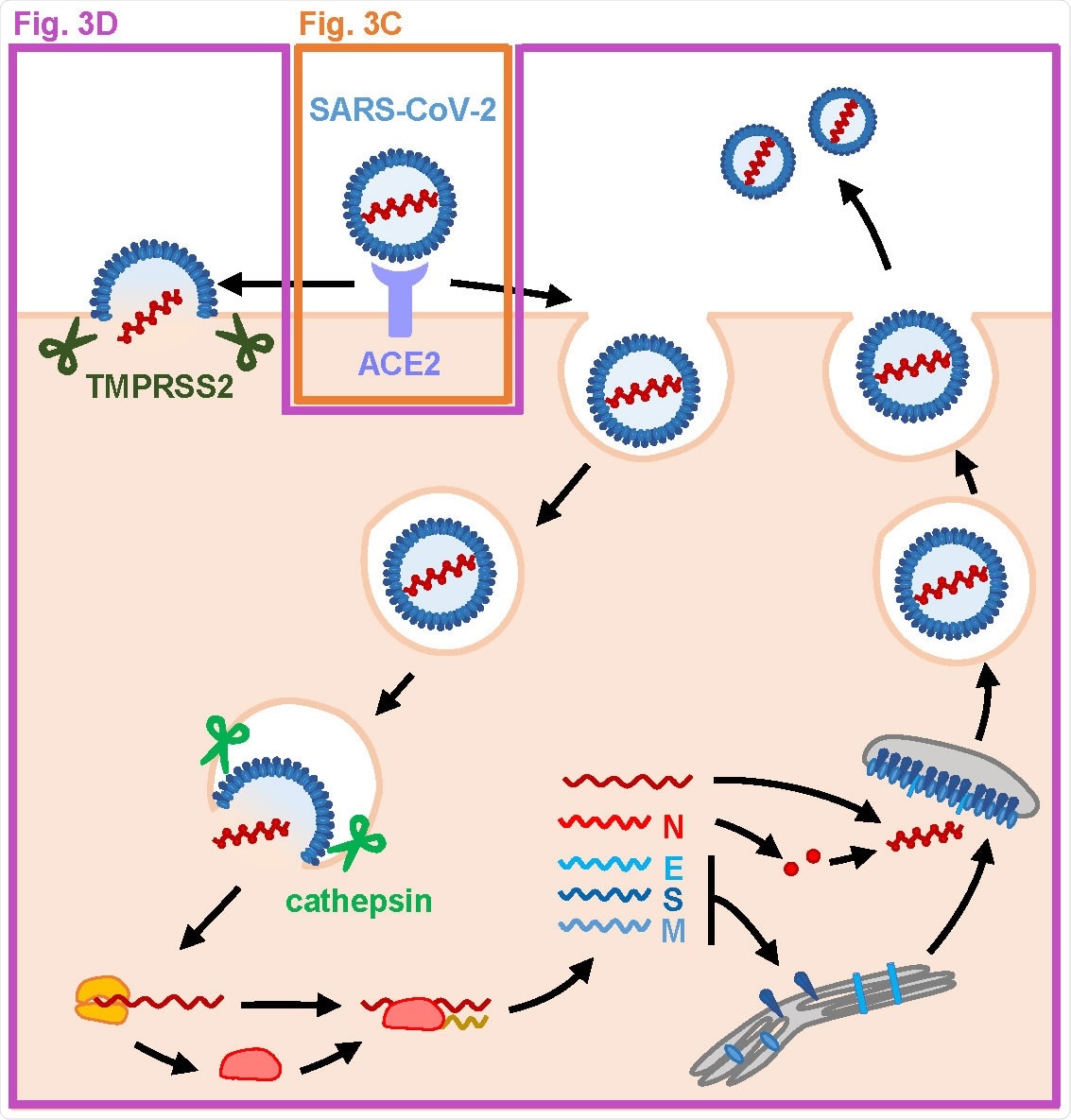
MFQ inhibits the SARS-CoV-2 entry process. (A) SARS-CoV-2 life cycle. SARS-CoV-2 infection is initiated with virus attachment to the host cells that involves the cellular receptor, angiotensin converting enzyme 2 (ACE2), followed by the cleavage of viral Spike (S) proteins by either transmembrane serine protease (TMPRSS2) on the plasma membrane or cathepsins in the endosome/lysosome that induces fusion of viral and host membranes. Viral RNA is translated, processed and replicated to be assembled into progeny virus with viral structural proteins and released extracellularly.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Important observations
They selected 27 anti-parasitic/anti-protozoal drugs for screening based on their demonstrated antiviral efficacies against RNA viruses and bioavailability in plasma. Using the cytopathic effect (CPE) assay, they identified an anti-malarial drug, mefloquine, as a potent anti- SARS-CoV-2 drug. Mefloquine, a derivative of hydroxychloroquine, successfully reduced the viral protein production and protected the cells from virus-induced damage.
The scientists compared the antiviral efficacy of mefloquine with other chloroquine derivatives and observed that mefloquine has the highest antiviral potency against SARS-CoV-2. A considerably higher hydrophobicity of mefloquine because of two trifluoromethyl groups may be responsible for such high antiviral efficacy.
To investigate whether mefloquine interferes with the viral entry phase or replication phase, they conducted the time-of-addition analysis, wherein drug candidates are administered at different time points to check specific activities. They observed that mefloquine, when applied at the viral entry phase, significantly reduced the viral RNA level. In contrast, administration of mefloquine after the initial round of viral entry showed much lower antiviral activity. These findings suggest that mefloquine may inhibit the entry of SARS-CoV-2 into host cells. With further analysis, they found that mefloquine inhibits the viral entry at the post-attachment phase. In other words, mefloquine prevents the viral entry probably by inhibiting proteolytic cleavage of spike protein, virus-host cell membrane fusion, and virus translocation to the replication site.
Keeping the possible added benefits of a combination drug therapy in mind, the scientists next examined the combined impact of mefloquine and nelfinavir, an anti-SARS-CoV replication inhibitor, on SARS-CoV-2 infected cells. They found that compared to mefloquine or nelfinavir treatment alone, the combined treatment with both mefloquine and nelfinavir further reduced the level of viral RNA. These findings indicate the potential synergistic effects of mefloquine and nelfinavir against SARS-CoV-2.
The scientists also used mathematical modeling to predict the antiviral efficacy of mefloquine in COVID-19 patients. Their prediction revealed that mefloquine has a long half-life (more than 400 hours) in plasma, sufficient to provide long-term protection. Moreover, about a 7% reduction in viral load and a significantly faster virus elimination were predicted by the mathematical analysis.
Taken together, the current study findings reveal that mefloquine, a hydroxychloroquine derivative, has significant antiviral activity against SARS-CoV-2, at least in vitro. Moreover, it has been found that the efficacy of mefloquine can be improved by administering it with viral replication inhibitors, such as nelfinavir.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources