Researchers in the United States have conducted a study demonstrating the potential of a novel therapeutic approach to treating coronavirus disease 2019 (COVID-19). The therapy augments the body’s T cell response to the causative agent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by providing a boost of rapidly expanded virus-specific T cells.
Tom Henley from Intima Bioscience and colleagues from Harvard Medical School, Harvard University, and Weill Cornell Medicine say these booster cells with enriched antigen-specificity may provide a pragmatic treatment for use in large-scale therapeutic settings.
“We demonstrate a novel technology platform for the robust and rapid expansion of virus-specific T cells as a potential cell therapy for COVID-19 and other viral pathogens,” writes the team.
Henley and colleagues say they will explore a timely regulatory path to assessing the technology in a phase I/II trial of patients infected with SARS-CoV-2, should the urgent need for treatments continue during the pandemic.
A pre-print version of the paper is available on the bioRxiv* server, while the article undergoes peer review.
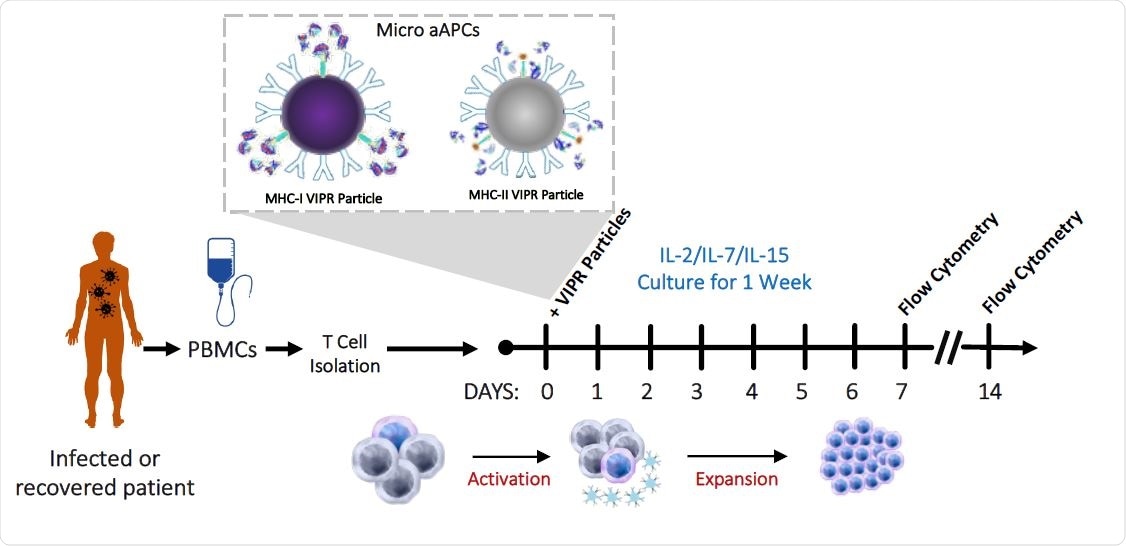
Detection and enrichment of antigen-specific Virus Induced Lymphocytes (VIL) in CMV infected individuals. a, Strategy for the isolation, stimulation and enrichment of CMV antigen-specific T cells from donor PBMCs.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Some patients require hospitalization for as long as a month
Clinical outcomes in cases of SARS-CoV-2 infection are highly variable. While the majority of infected individuals experience mild or even no symptoms, some patients develop severe disease that requires hospitalization for as long as a month.
“Validated therapies are few and largely supportive or non-specific, such as the use of dexamethasone to delay mechanical ventilation in COVID-19 induced pulmonary failure,” say the researchers. “Thus, there is an urgent need for specificity and efficacy in the clinic to mitigate disease progression and severity.”
Therapies that may be able to improve disease outcomes would need to be quickly administered during the critical time window of disease progression prior to or early on during intubation and respiratory support, they add.
How adoptive cell therapies might help
Adoptive cell therapies utilizing patient-derived tumor-infiltrating lymphocytes (TILs) have been shown to generate reproducible clinical responses in the setting of highly immunogenic tumors such as melanoma.
The efficacy of this therapy relies on specificity to the cancer antigen and on the ability to expand a large quantity of antigen-specific T cells ex vivo that can be administered to the patient to induce the lysis of tumor cells.
Similarly, antigen-specific T cells are an essential component of an effective cellular immune response to viral infection, and activation of cytotoxic T cells clears infection by killing virus-infected cells.
The researchers say protective virus-specific T cell therapy could potentially serve as a treatment for SARS-CoV-2 and other pandemic viral pathogens.
“Thus, we propose a similar-in-principle novel antigen-specific adoptive T cell therapy for viral infection predicated on precise T cell viral antigen-specificity,” said Henley and colleagues. “However, this viral platform requires cell kinetic expansion in days not weeks, thus allowing for the adoptive transfer of immunologically competent T cells in a therapeutically relevant time course in the setting of acute viral infection.”
What did the researchers do?
Henley and team developed an adoptive T cell therapy for COVID-19 based on rapid ex vivo expansion of SARS-CoV-2 antigen-specific virus-induced lymphocyte (VIL).
The researchers designed a T cell expansion platform comprising of microbead-based artificial antigen-presenting cells (aAPCs) carrying major histocompatibility complex pentamer-peptide complexes, specific for immunodominant SARS-CoV-2 epitopes.
The platform was used to expand virus-specific VIL derived from the peripheral blood mononuclear cells of recovered COVID-19 patients.
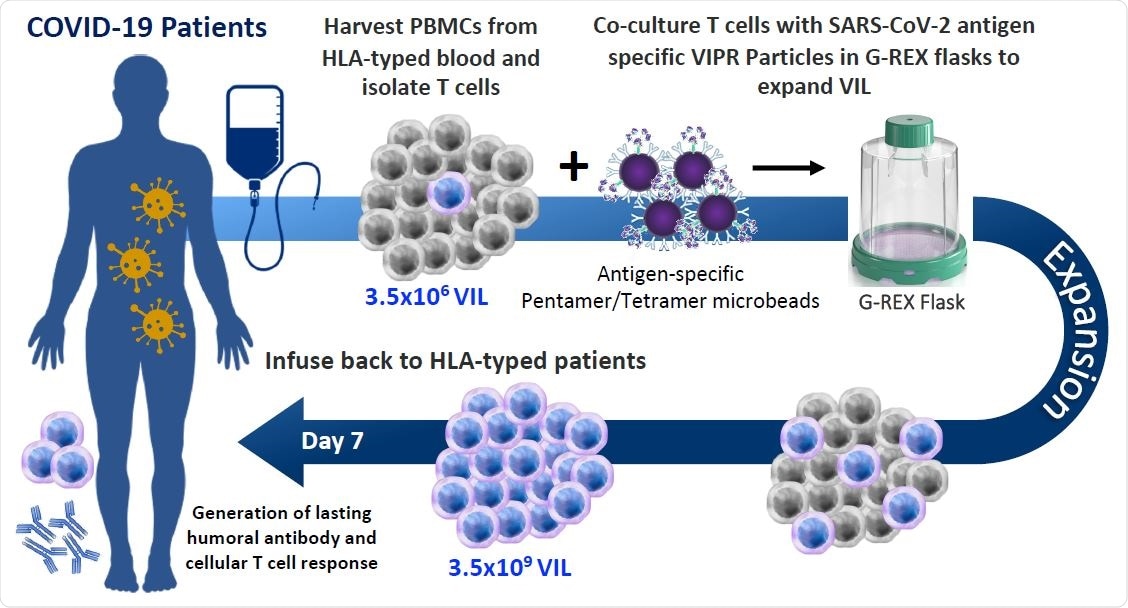
Allogeneic VIL Therapy Platform: An adoptive cell therapy for the treatment of individuals suffering from severe symptoms of COVID-19. a, Schematic for a COVID-19 cell therapy in which PMBCs are collected from the blood of HLA-typed hospitalized patients and total T cells isolated. T cells are stimulated with HLA-matched MHC-I/MHC-II antigen-specific SARS-CoV-2 VIPR beads to enrich and expand CD4+ and CD8+ T cells with TCRs specific for the SARS-CoV-2 antigen epitopes. These antigen-specific VIL expand at an average of 1,000-fold prior to adoptive transfer back to HLA-matched patients to mediate a T cell immune response to support the eradication of the SARS-CoV-2 virus and to engender protective immunity against repeat infection.
What did the study find?
The aAPCs expanded VIL up to 1,000-fold within just 7 days of T cells being isolated from patients.
The expanded VILs are enriched in virus antigen-specificity, demonstrate polyfunctional cytokine responses and acquire a T effector memory phenotype that may contribute to a robust immune response, reports the team.
What are the study implications?
The researchers say the study has shown that this novel technology platform can be used for the robust and rapid expansion of virus-specific T cells as a potential therapy for COVID-19.
They also say the platform would provide a modality for the expansion of any clinically relevant virally-mediated disease where an immune-boosting dose of virus-specific T cells would aid in viral clearance and improving disease outcome.
“We will explore a timely regulatory path to evaluating the VIL platform technology in a phase I/II clinical trial of SARS-CoV-2-infected patients, should there continue to be an urgent need during the course of the COVID-19 pandemic,” concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Henley T, et al. A Novel Cell Therapy for COVID-19 and Potential Future Pandemics: Virus Induced Lymphocytes (VIL). bioRxiv, 2020. doi: https://doi.org/10.1101/2020.11.26.400390, https://www.biorxiv.org/content/10.1101/2020.11.26.400390v1
- Peer reviewed and published scientific report.
Sivapalan, Rohan, Jinyan Liu, Krishnendu Chakraborty, Elisa Arthofer, Modassir Choudhry, Philip S. Barie, Dan H. Barouch, and Tom Henley. 2021. “Virus Induced Lymphocytes (VIL) as a Novel Viral Antigen-Specific T Cell Therapy for COVID-19 and Potential Future Pandemics.” Scientific Reports 11 (1): 15295. https://doi.org/10.1038/s41598-021-94654-y. https://www.nature.com/articles/s41598-021-94654-y.