Early on in the COVID-19 pandemic, it became clear that the elderly population over 60 years of age was at greater risk from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Some scientists have attributed this to the increased prevalence of chronic underlying disease in this age group, which poses an independent risk factor for adverse outcomes in COVID-19. A new study published on the preprint server bioRxiv* in December 2020 reveals the role played by certain genetic factors in this group, in particular the FASLG gene belonging to the tumor necrosis factor (TNF) family.
The SARS-CoV-2 virus causes an asymptomatic or mild infection in most cases. In those patients with more severe illness, it affects the lung parenchyma, primarily resulting in edema of the lung alveoli, the deposition of fibrin, and hemorrhage. Vascular changes are characteristic of this condition, manifesting in some as thrombosis of the microvasculature, leading to intravascular coagulation, and, in the critically ill, multi-organ failure.
The fact that many severe COVID-19 patients display signs of disrupted coagulation, including heart attacks, pulmonary embolism, and thrombosis, causing increased death rates following the infection, is concerning. These changes are associated with inflammatory dysregulation, leading to a cytokine storm. The hyper-intense levels of pro-inflammatory mediators activate immune cells and platelets and causes hyperactivation of the coagulation cascade.
They examined the blood transcriptomics data during aging from 670 males and females in the Genotype-Tissue Expression (GTEx) database. They aimed to identify the differentially expressed genes (DEGs) in whole blood in COVID-19 patients aged 20-79 years, with particular reference to the genes that translated into proteins that interact with viral proteins.
They then explored the DEG-related SARS-CoV protein-protein interactions (PPI) because of the close similarity between this virus and SARS-CoV-2. This was followed by gene ontology studies to gain insights into the function of the DEGs and PPIs, to better understand the association between these PPIs and the pathogenesis and potential druggability of the disease.
The samples were stratified by age group, in the following subsets: 30-39, 40-49, 50-59; 60-69 and 70-79. Each of these groups was compared with the age group 20-29 years. To be considered a DEG, a log2 magnitude or more significant change was required, with a false discovery rate (FDR) < 0.05.
They found a unique gene expression pattern in the youngest age group. There were 22 relevant DEGs in blood samples from older patients. The number of DEGs and PPIs was found to increase with age seen from the age of 50 onwards, with 62, 251, and ~900 DEGs in the age groups of 50-59, 60-69, and 70-79, respectively.
In the 50-79 pooled age group, five genes, namely, FASLG, CTSW, CTSE, VCAM1, and BAG3, were overexpressed.
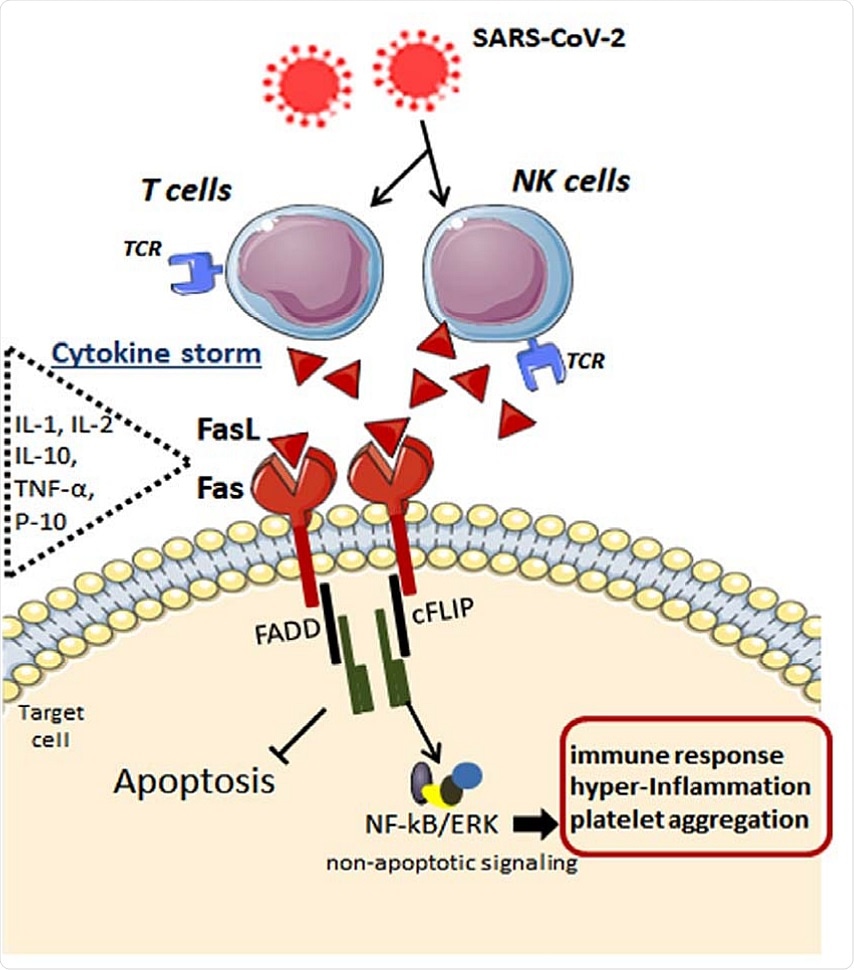
Possible mechanisms underlying the Fas-FasL-mediated signaling in the immune cells after SARS-CoV-2 infection. By infecting specific immune cells, the cytokine storm takes place with elevation in interleukins and chemokines such as IL-1, IL-2, IL-10, IFNs, TNF-α, IP-10, like others. In addition to inflammatory mediators, FasL ligand is highly released by these immune cells, which, in turn, bind to its Fas receptor in the target cell. Upon FasL binding, the apoptosis inhibitor cFLIP is upregulated at the post-translational level and is associated with TRAF1 and TRAF2 and with the kinases RIP and Raf-1, resulting in activation of NF-kB transcription factor and ERK signaling. These anti-apoptotic signals lead to potentiated pro-inflammatory effects via Fas engagement, while FasL increases activated human T cells’ proliferation. Fas, death receptor; FasL, Fas ligand (tumor necrosis factor ligand superfamily member 6); NK, natural killer; T, type of lymphocyte; TCR, T-cell receptor; IL-1, interleukin1; IL-2, interleukin2; IL-10, interleukin 10; TNF-α, tumor necrosis factor alpha; IP-10, interferon gamma-induced protein 10; FADD, FAS-associated protein with death domain; cFLIP, FADD-like IL-1β-converting enzyme-inhibitory protein; NF-kB, nuclear factor kappa B; ERK, extracellular signal-regulated kinase.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Functional Relevance
FASLG is overexpressed in both sexes after the age of 50, but the cathepsin genes, CTSW and CTSE are high after 60, along with the adhesion molecule VCAM1 and the chaperone regulator molecule (BAG3). The PPI network of these enriched targets showed FASLG, SRC, VCAM1, BAG3, and HSPA5 to be primarily involved in interactions between the virus and immune components, inflammation, platelet activation and aggregation. Interestingly, all these were linked to the plasma membrane in some way.
A gene ontology (GO) analysis was then carried out in samples from aged individuals to understand how these changes affected host function. They found the closest functional relevance was with immune function, subcellular organization, adhesion of cells, signaling and stimulus-response, biological regulation, and development.
The CTSW and CTSE proteins were related to viral endosomal exit, but the other three were found to interact with ORF8, and perhaps with the spike protein.
FASLG and CTSW gene expression was linked to NK cells, CD8+ T cells, and memory T cells. CTSE expression was higher in males above 50 compared to females. CTSE is expressed on immune cells that bear MHC-II antigens and might be a potential therapeutic target in COVID-19.
VCAM1 and BAG3 showed age-dependent expression, the latter mainly on CD4+ T cells, naive T cells, and CD14+ monocytes. These genes are related to virus-host cell interactions via the spike protein, allowing viral entry into the bloodstream. The resulting increase in SRC and HSPA5 in those aged 79-79 causes platelet aggregation and activation, and this, in turn, may trigger the coagulation cascade.
VCAM1 was unique in showing increased transcription levels in individuals aged 60-69 relative to those aged 20-29, in both sexes. This being an endothelial adhesion marker, it may correlate with the presence of diffuse endothelial inflammation and infection of endothelial cells, with thromboembolism, all directly correlated with the severity of disease and coagulopathy.
BAG3 expression was predominant for CD4+ memory T cells, naïve T cells and CD14+ monocytes. These blood cells did not significantly express VCAM1 and CTSE genes.
Age-Related DEGs and Cytokine Storm
In other viral illnesses, including SARS and MERS, and influenza, cytokine monitoring can reduce the mortality rate. However, in severe COVID-19, interferon levels drop, these being antiviral factors, while interleukins rise to high levels, along with other pro-inflammatory molecules – TNF, IL-6, IL-1β and some chemokines such as CCL-2 and -3.
In fact, the gene expression profile in the oldest group is inversely related to that in the youngest group. In individuals over 50 years of age, inflammatory and immune pathways were activated most highly. This finding is significant since severe and critical COVID-19 is thought to be mediated by this surge in cytokines causing systemic inflammation.
Cytokines are carried at high levels throughout the body by the blood, and this may mediate multiple organ dysfunction.
FASLG in NK and CD8+ T Cells Drives Hypercytokinemia
Fas ligand, FasL, is a transmembrane homotrimer, part of the same family as TNF. The Fas/FasL complex formation triggers a signaling pathway that results in the assembly of the death-inducing signaling complex (DISC) within the cell. The outcome is the death by apoptosis of immune cells and of infected cells, along with the apoptosis-independent induction of severe inflammation.
This complex may activate T cells and NK cells that come into contact with SARS-CoV-2 during the stage of viremia. The outcome may be both an intensified immune response and exhaustion of the activated blood cells. By promoting the incorporation of cFLIP to the DISC, FasL results in the activation of the transcription factors NF-kB and ERK/AP-1. This is also promoted by IL-8 within the cells of the bronchiolar epithelium. Macrophages secrete TNF-α when bound to FasL. FasL in turn, causes IL-2 release and T cell proliferation.
FasL was found to be derived chiefly from CD8+ T cells and NK cells in COVID-19 patients, and might be among the array of host proteins with which the viral ORF8 interacts. This viral protein is secreted in large amounts and reduces MHC-I expression on cells. This may be why the virus spreads so rapidly and eludes immune recognition and clearance. Fas/FasL signaling also impacts endothelial function and neutrophil lifespan and apoptosis of infected cells, along with potential viral replication. Thus, this may be a potential therapeutic target.
The ligation of Fas on CD8+ T cells can cause their differentiation and activation, which may explain their low count in these patients. This may also, along with the corresponding overactivation of macrophages and CD4+ T cells, drive the cytokine storm. Both immune and inflammatory cells have been found to infiltrate the patient’s lung tissues in COVID-19, and IL-6 is high in critically but not moderately ill patients.
Implications
Some studies indicate that the altered expression of DEGs in older individuals is a reflection of existing dysregulation, which is exacerbated by the virus, and that this accounts for the worse outcomes. The authors conclude, “The increased expression of FASLG in blood during aging may explain why older patients are more prone to severe acute viral infection complications. Because hypercytokinemia is described as the framework for disease severity and high-risk death, we highlight FASL as a prognostic biomarker and a therapeutic proposal to modulate inflammation in elderly patients with COVID-19.”
Source

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Chuffa Sr. L. G. et al. (2020). The aging whole blood transcriptome reveals a potential role of FASLG in COVID-19. bioRxiv preprint. doi: 10,000,000, https://doi.org/10.1101/2020.12.04.412494, https://www.biorxiv.org/content/10.1101/2020.12.04.412494v1
- Peer reviewed and published scientific report.
Almeida Chuffa, Luiz Gustavo de, Paula Paccielli Freire, Jeferson dos Santos Souza, Mariana Costa de Mello, Mário de Oliveira Neto, and Robson Francisco Carvalho. 2021. “Aging Whole Blood Transcriptome Reveals Candidate Genes for SARS-CoV-2-Related Vascular and Immune Alterations.” Journal of Molecular Medicine 100 (2): 285–301. https://doi.org/10.1007/s00109-021-02161-4. https://link.springer.com/article/10.1007/s00109-021-02161-4.