Early on in the ongoing coronavirus disease 2019 (COVID-19) pandemic, the antibiotic azithromycin was regarded as being a potentially highly effective drug against the virus that causes it, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Hundreds of thousands of doses have been dispensed on this basis to treat these patients. Evidence in support of this hypothesis has been lacking, however.
Now, a randomized controlled trial from the UK provides, in its preliminary findings, that it offers no clinical benefit at all, and should be used only for standard indications, that is, if there is any evidence of bacterial infection susceptible to this antibiotic. The study was published in December 2020 in the preprint server medRvix*.
In the first wave of the pandemic in the UK, over a quarter of individuals reported died from the infection. Among those who required invasive mechanical ventilation, more than 37% died. A major contributor to this excess mortality is the hyper-inflammatory process that is triggered by the host immune process as it senses the presence of the virus.
The result includes acute pneumonia with widespread damage to the lung alveoli, inflammatory cells infiltrating the lung tissue and causing further destruction, and thrombosis of microvascular vessels.
The Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial included azithromycin, a broad-spectrum antibiotic, and immunomodulator, among its panel of therapies for evaluation. Several potential treatment modalities were compared in UK patients with COVID-19 who were hospitalized. These included azithromycin, dexamethasone, hydroxychloroquine, and lopinavir-ritonavir, tocilizumab, convalescent plasma, REGEN-COV2 (a combination of two anti-SARS-CoV-2 spike monoclonal antibodies), aspirin, and colchicine.
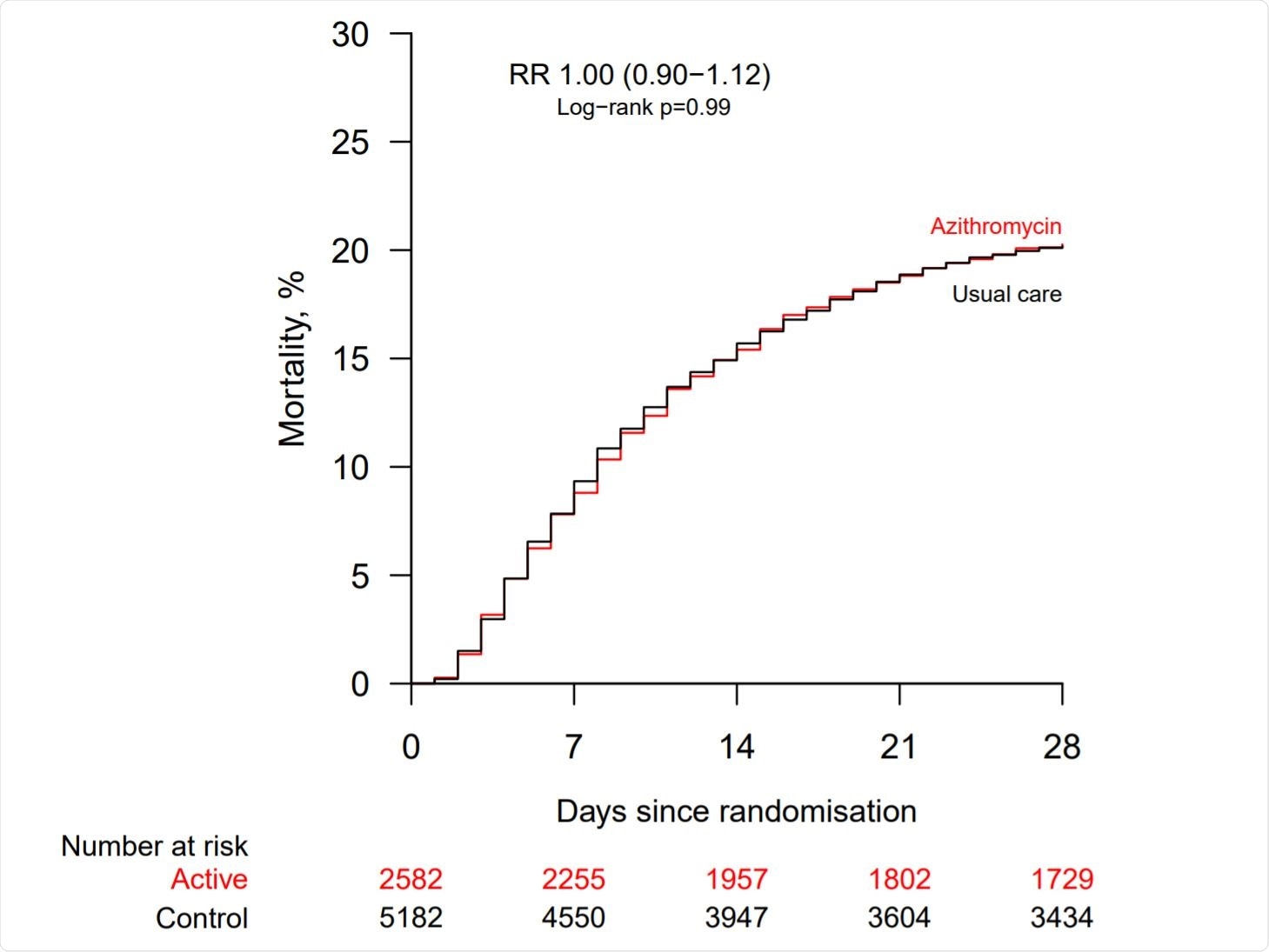
Effect of allocation to azithromycin on 28−day mortality. Image Credit: https://www.medrxiv.org/content/10.1101/2020.12.10.20245944v1.full.pdf

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Immunomodulators in COVID-19
Potent immunomodulators such as dexamethasone are of use in patients with hypoxia due to COVID-19 pneumonia. Other drugs thought to be of possible use in this category include azithromycin and hydroxychloroquine. They inhibit the activation of neutrophils and the secretion of pro-inflammatory cytokines. For this reason, azithromycin has been in wide use in bacterial pneumonia and chronic inflammations of the lung. Azithromycin has demonstrated antiviral activity against SARS-CoV-2, among other viruses, in vitro.
When used in pneumonia associated with the flu, macrolides have been found to accelerate the fall in inflammatory parameters.
Study Details
The current study assigned patients randomly to usual care or usual care plus azithromycin in a ratio of 2:1, with the patients being aware of their treatment protocol. Patients in the azithromycin arm received either 500 mg by mouth, nasogastric tube, or intravenously once a day for 10 days, or until discharged from this trial if sooner.
Follow-up was carried out once, at 28 days from randomization, or after discharge, or after death, whichever was soonest. This period is still ongoing for over a quarter of patients, whose data is therefore not included in this preliminary report.
The researchers looked chiefly at mortality from all causes. They found that of over 2,500 patients allocated to azithromycin vs ~5,100 allocated to usual care, a follow -up was available for 5,910 patients. Approximately 90% of patients in the azithromycin group received one or more doses, and 92% received any macrolide. In the usual care group, 1% and 15% received one or more doses, and any macrolide, respectively.
The median period of azithromycin treatment was 6 days. Other treatments given to these patients, in both arms, included a corticosteroid, remdesivir, and convalescent plasma (CP).
No Change in Mortality or Ventilation
The researchers found no difference in deaths, which occurred in approximately a fifth of patients in both groups. The same results were obtained when the groups were stratified further by age, sex, ethnic origin, type of respiratory support, days since the onset of symptoms, corticosteroid use, and predicted risk of death in 28 days.
The time to discharge alive from hospital was also the same in both arms, at a median of 12 days in the azithromycin group vs 13% in the usual care group. The probability of discharge was ~60% for both groups. Among patients who were not on mechanical ventilation at baseline, the risk of requiring ventilation or of death was again similar in both arms, at 21% and 22%, respectively.
Again, there was no difference in cause-specific mortality, successful cessation of ventilation, need for dialysis or hemofiltration, nor in the frequency of new abnormalities of heart rhythm.
What are the Implications?
The results of this large randomized trial show that azithromycin is not an effective treatment for patients hospitalized with COVID-19.”
Since the concomitant use of a corticosteroid did not change the outcomes, they also suggest that the immunomodulatory effects of azithromycin are either inadequate to modify the clinical course of the disease or are off-target in this condition.
Macrolide antibiotics are a commonly used mode of treatment of lower respiratory tract bacterial infections. In over 75% of hospitalized COVID-19 patients, antibiotics are prescribed, typically to prevent bacterial superinfection. Surprisingly, the study also shows that with moderate or severe COVID-19, where secondary bacterial infection of the lungs might have been expected, azithromycin produced little clinical benefit.
The possibilities then are that either the rate of such infection is low in COVID-19, or that the effect of azithromycin in treating such infections was overshadowed by that of other antibiotics in extensive use at the same time, especially the penicillins and other beta-lactams. Of course, the trial does not look at its putative benefits in early or mild COVID-19.
The researchers also indicate that the indiscriminate use of azithromycin could cause harm at a widespread level, by inducing the emergence of antibiotic resistance. Since this drug is already classified in the WHO Watch Group of Antibiotics as one with high resistance potential and is on the priority list for antimicrobial stewardship, the study underlines the poor support for its extensive use in COVID-19, and indeed, for the use of antibiotics in general.
The RECOVERY trial had adequate patient enrolment to allow even modest patient benefits to be detected, unlike earlier trials like COALITION I and COALITION II. Several other trials of macrolide efficacy in COVID-19 patients are ongoing.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Horby, P. W. et al. (2020). Azithromycin in Hospitalised Patients with COVID-19 (RECOVERY): a randomized, controlled, open-label, platform trial. medRxiv preprint. doi: https://doi.org/10.1101/2020.12.10.20245944. https://www.medrxiv.org/content/10.1101/2020.12.10.20245944v1
- Peer reviewed and published scientific report.
Abaleke, Eugenia, Mustafa Abbas, Sadia Abbasi, Alfie Abbott, Ashraf Abdelaziz, Sherif Abdelbadiee, Mohamed Abdelfattah, et al. 2021. “Azithromycin in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial.” The Lancet 397 (10274): 605–12. https://doi.org/10.1016/s0140-6736(21)00149-5. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00149-5/fulltext.