The virus attaches to host cells via the trimeric spike glycoproteins that stud its lipid envelope. The spike attaches to the host cell receptor, the angiotensin-converting enzyme 2 (ACE2), via its receptor-binding domain (RBD). The binding of the spike to the receptor causes a conformational change in the virus spike that triggers membrane fusion between the virus and the cell membrane.
This fusion mediates the formation of a multinucleated or syncytial cell. This fusion event is important in many infections such as respiratory syncytial virus (RSV), and human immunodeficiency virus (HIV), but their role in SARS-CoV-2 infection is unclear.
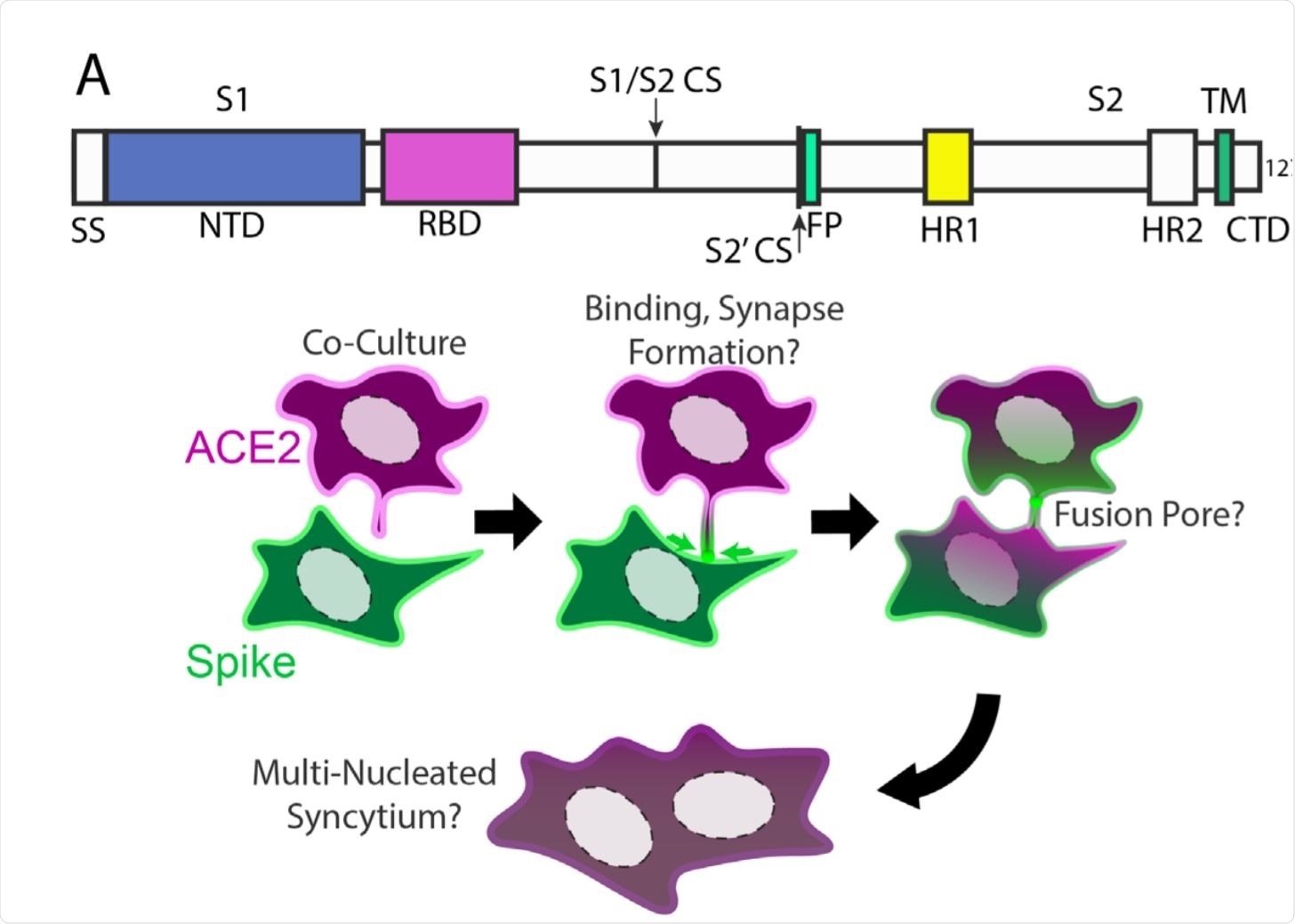
Syncytia derive from fusion events at synapse-like, spike-ACE2 protein clusters. Image Credit: https://www.biorxiv.org/content/10.1101/2020.12.14.422737v1.full.pdf
The stepwise fusion of virus-host cells
The current study focuses on the mechanisms underlying syncytial formation, using a microscopy-based approach. The researchers used human osteosarcoma cells expressing either ACE2 or full-length spike/RBD. A fluorescent tag was added to enable easy detection.
Concerning the mechanism of syncytia formation, they postulated that syncytia might form step by step. In confirmation, they found that when the ACE2 and RBD-expressing cells were cultured together, ACE2-RBD complexes were found at points of cell contact.
On the other hand, when ACE2 and spike-expressing cell types were co-cultured, almost immediately they observed the accumulation of dozens of clustered ACE2-full-length spike protein complexes, near points of intercellular contact. These were formed by the accumulation of spike protein near protruding membrane foci containing ACE2. These structures persisted for several minutes, similar to synapses formed between nerve or immune cells.
This initial punctate fusion at multiple points led to the eventual formation of cellular fusion pores. The spike cluster at each point was drawn towards the center of the ACE2-expressing cell within minutes or longer periods. Dilated fusion pores were observed, indicating that cytoskeletal actions may act along with endocytosis, or independently, to stabilize the mixing of the two lipid bilayers, of the viral and host cell membranes.
Cytopathic vacuolization
On densely plated cell cultures, these events are completed within an hour, and small syncytia join to form larger syncytia within a day, for most of the cells. Vacuoles form within the syncytial over time, probably following the collapse of the cell organelles following fusion. By 72 hours or less, the cells break down by autophagy, leaving stationary vesicles coated with a spike or ACE2 protein.
Syncytia formation followed by the vacuolization of infected cells may account for the widespread lung damage seen in severely ill COVID-19 patients.
Intriguingly, SARS-CoV-2 spike is a particularly potent mediator of syncytia formation relative to both SARS-CoV-1 spike and commonly studied fusogens.”
Indeed, they found a very high incidence of syncytia in patients who had died of COVID-19-induced diffuse alveolar damage (DAD), but not in patients who tested positive for the virus but did not show pulmonary involvement, nor in patients who died of DAD before the start of the pandemic.
However, the viral nucleocapsid (N) protein was not detected within the syncytia. “We cannot rule out a yet-to-be-identified pulmonary abnormality specific to SARS-CoV-2 infection.”
Proxy for viral entry and cellular fusion
This cell system is a faithful representation of post-receptor binding events. When spike-expressing cells interact with Vero E6 cells and other cells that support SARS-CoV-2 infection, cell fusion occurs.
The same domains were involved in both viral entries into the host cell and for cell-cell fusion. Both required the presence of the S2’ cleavage site, but the S1/S2 interface was not necessary. And finally, they found that several different fluorophores could be used to mark this event.
As a result, they were able to use this co-culture system instead of the far more dangerous live SARS-CoV-2, dispensing with the biosafety level 3 precautions.
Screening potential therapies
To confirm this, the researchers conducted a high-throughput screen of compounds that modulate fusion between cells, including over 6,000 chemicals and 30 spike variants. They built and tested three different methods, two using mCherry and GFP markers on the spike and ACE2-expressing cells, to indicate a fusion of the cells carrying each marker by nuclear co-localization of mCherry/GFP. The third used split-GFP that requires its two halves to come together, as after cell fusion, to produce fluorescence.
The results showed that among protease inhibitors, only nelfinavir was capable of blocking fusion, perhaps because it targets processes other than proteolysis. No single route of endocytosis was found to be an essential part of syncytia formation. However, certain specific drugs did show the ability to suppress this event, including one which prevented actin polymerization.
Several effective drugs were involved in membrane lipid disruption, notably the statins, dicholorophenethyl-imidazoles, and tetrahydropyran-containing macrocyclic lactones. Importantly, these can all have direct effects on the plasma membrane.
Further work showed that the proximal part of the spile protein, namely, the transmembrane domain, probably has an essential role in synapse formation with ACE2-bearing cells.
The researchers also found that the post-translational addition of palmitoyl residues to the cysteines in the cytoplasmic domain of the spike protein is also important to fusion. Palmitoylated membrane proteins are preferred by cholesterol for the formation of lipid rafts, or lipid-rich membrane nanodomains
What are the findings and implications?
The observations suggested that the spike protein interacted with membrane lipids rather than membrane proteins. However, it did not accumulate within lipid rafts (membrane domains rich in cholesterol), an unexpected finding given the conventional involvement of sphingomyelin-enriched membrane nanodomains in such interactions.
Instead, the spike directly associates with cholesterol molecules outside such rafts. Finally, they found that cholesterol supplementation in the region of interaction markedly boosts fusion.
From these findings, it seems that many drugs that inhibit fusion do so by removing cholesterol from the plasma membrane. Since this involves the plasma membrane of the virus, containing the spike protein, though derived from the host cell, these drugs are not expected to be useful in preventing viral entry. However, lipid-targeting drugs could prevent the formation of virus particles that can produce fusion.
One promising drug, Apilimod, was able to prevent such entry at nanomole concentrations because it inhibits the lipid kinase PIKFYVE. Another compound, MBCD, that removes plasma membrane cholesterol directly, also prevented viral entry via direct fusion, in an earlier experiment involving spike-bearing pseudoviruses and cells expressing ACE2/TMPRSS2.
In a further experiment, treating cells with MBCD before SARS-CoV-2 exposure failed to prevent infection, though syncytia were not formed. However, when the virus was exposed to this agent, the infection was completely inhibited. “Therefore, the cholesterol content of SARS-CoV-2 viral particles, but not the host cell, is critical to infectivity.”
A further interesting observation was that the top 4 human transmembrane proteins with high cysteine and aromatic content in the membrane-proximal domains, similar to the SARS-CoV-2 spike protein, were central to the formation of tight junctions between cells. Thus, the same type of mechanism may underlie adhesion and cell-cell contact between SARS-CoV-2 and host cells.
Our results suggest that modulation of membrane composition may inhibit viral propagation, and further informs critical lipid-protein assemblies in physiological syncytia and cell adhesion.”
In fact, earlier retrospective studies have shown that COVID-19 patients on cholesterol-lowering statins have significantly lower death rates. This could also account for several other COVID-19 risk factors like obesity, advanced age, and menopause, which are linked to abnormal lipid metabolism.
“Additional work is clearly required, but in the context of the rapidly evolving landscape of COVID-19 treatment options, our findings underscore the potential utility of statins and other lipid-modifying treatments.”