As a part of the COMMUNITY (COVID-19 Immunity) study, researchers from Sweden reveal long-lasting IgG neutralizing antibody response against spike glycoprotein of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the vast majority of convalescent individuals – even after asymptomatic or mild infection. Their results are currently available on medRxiv* preprint server.
The expeditious spread of SARS-CoV-2, a causative agent of coronavirus disease (COVID-19), is facilitated by a considerable portion of asymptomatic and pre-symptomatic viral transmission. This is why it is crucial to fully understand the functionality and durability of the immune response against this virus.
It is known that antibodies targeting different virus-encoded proteins are key players in conveying protective immunity against SARS-CoV-2. Among the prime targets are spike glycoprotein, which facilitates viral access to the host cell, as well as the highly abundant and conserved nucleocapsid protein.
Nonetheless, the duration, kinetics, and efficacy of circulating SARS-CoV-2 antibodies are less familiar, mostly because we are dealing with a new virus. There are actually conflicting studies that show either a rapid decline in circulating IgG antibodies within a few weeks after COVID-19 or their detection up to six months following symptom onset.
Hence, understanding the long-term humoral response (which includes virus neutralization capacity) in asymptomatic to mild SARS-CoV-2 infections is pivotal in estimating the immune response on a population basis, possible risk of reinfection, as well as vaccine responses.
This is why a research group from Sweden, led by Dr. Sebastian Havervall from the Department of Clinical Sciences at Karolinska Institute in Danderyd Hospital (Stockholm), decided to appraise the duration and efficacy of circulating antibodies four months after infection in a large cohort of patients (primarily healthcare workers).
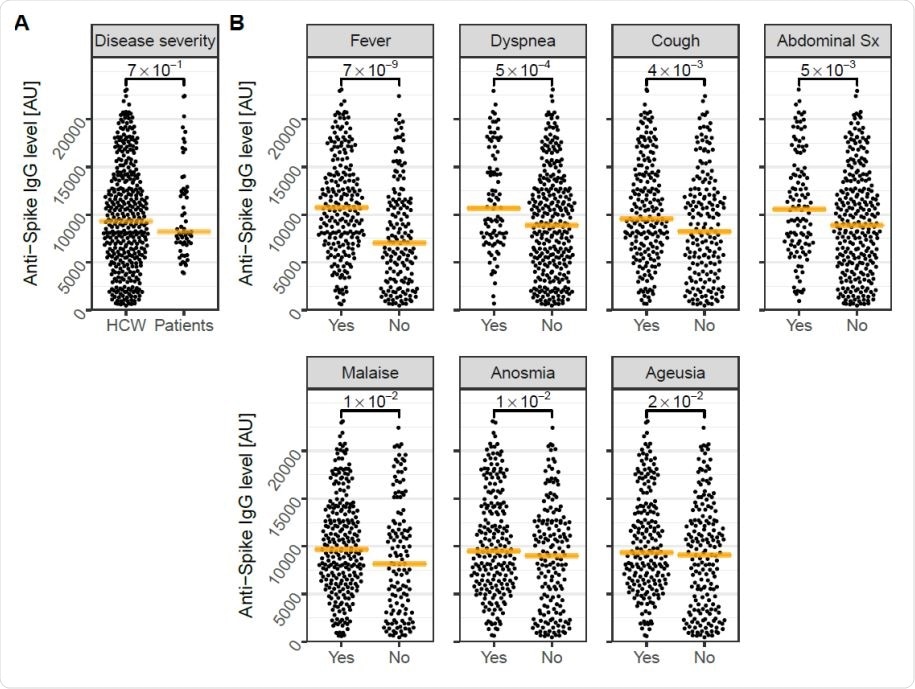
Four-month follow-up levels of anti-Spike IgG are associated to COVID-19 symptoms A) Four-month follow-up levels of anti-spike IgG did not differ between HCW and convalescent COVID-19 patients, but were B) significantly increased in HCW with selfreported fever, dyspnea, cough, abdominal symptoms, malaise, anosmia, or ageusia prior to study inclusion. Orange lines depict the median. P-values are shown with brackets. Sx; symptoms. AU: Arbitrary Units.
A long-term immunity study
This longitudinal cohort study included a total of 1972 healthcare workers, with 386 of them exhibiting antibodies against the SARS-CoV-2 spike glycoprotein antigen at study inclusion. It was conducted as a part of the COMMUNITY (COVID-19 Immunity) study, which is an ongoing investigating of long-term immunity after COVID-19 in Stockholm, Sweden.
Venous blood samples were taken at study inclusion and after four months of follow-up. Furthermore, IgG reactivity was measured towards spike glycoprotein trimers entailing the prefusion-stabilized spike ectodomain and the C-terminal domain of the nucleocapsid protein.
Microneutralization assay was utilized, and cells were inspected by optical microscopy after four days. In cases when less than 50% of the cell layer showed signs of the cytopathogenic effect (i.e., structural changes of the host cell due to viral invasion), the well was regarded as neutralizing.
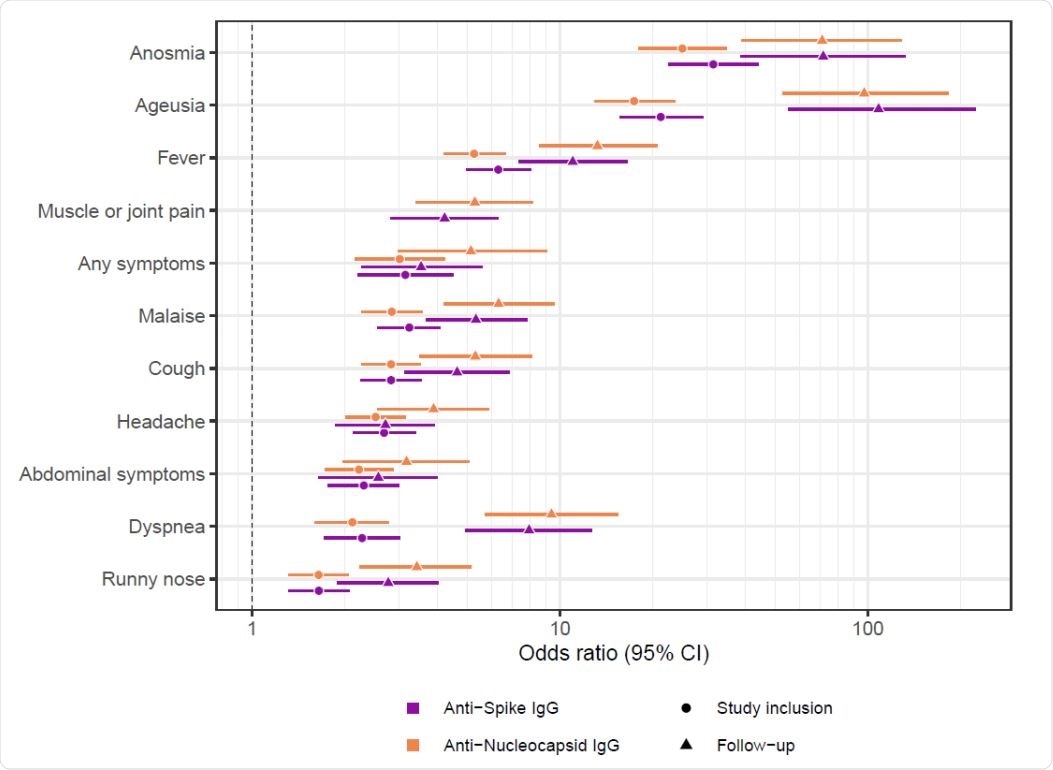
Seroconversion was associated with prior COVID-19 symptoms Seroconversion to anti-spike IgG (purple) and anti-nucleocapsid IgG (orange) prior to study inclusion (circles) and during the follow-up period (triangles) was associated with self-reported anosmia, ageusia, fever, muscle or joint pain, presence of any symptoms, malaise, cough, headache, abdominal symptoms, dyspnea, or runny nose. CI: Confidence Interval.
Highly persistent neutralizing antibodies
In short, this study has shown that the vast majority of healthcare workers (98% of them) remained seropositive for SARS-CoV-2 anti-spike IgG at least four months after the infection, regardless of having mild symptoms or no symptoms at all. Moreover, antibody levels were no different from 59 convalescent patients suffering from severe to critical COVID-19.
The researchers have also corroborated earlier findings of strong concordance between SARS-CoV-2 anti-spike IgG levels and virus neutralization capacity – supporting, in turn, an enduring immunity after COVID-19 even in asymptomatic individuals or those with mild symptoms.
Conversely, anti-nucleocapsid IgG levels declined to an undetectable range in 32% of the study participants with mild infection (or without symptoms), emphasizing the importance of diligent antigen selection for any test or survey.
Implications of the findings
"Taken together, our findings imply a strong and long-lasting humoral immune response against SARS-CoV-2, even after asymptomatic or mild infection", conclude the authors of this medRxiv paper.
Furthermore, demonstrated differences in antibody kinetics in dependence on the antigen is basically an argument against the use of the nucleocapsid protein as a possible target antigen in population-wide SARS-CoV-2 serological surveys and analyses.
Everything said, these findings are indeed highly relevant for understanding correlates of long-term humoral immunity after SARS-CoV-2 infection, which is necessary for public health planning, the evaluation of potential reinfection risk, as well as for long-term vaccine response appraisal.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Havervall, S. et al. (2020). SARS-CoV-2 induces a durable and antigen-specific humoral immunity after asymptomatic to mild COVID-19 infection. medRxiv. https://doi.org/10.1371/journal.pone.0262169, https://www.medrxiv.org/content/10.1101/2021.01.03.21249162v1
- Peer reviewed and published scientific report.
Havervall, Sebastian, August Jernbom Falk, Jonas Klingström, Henry Ng, Nina Greilert-Norin, Lena Gabrielsson, Ann-Christin Salomonsson, et al. 2022. “SARS-CoV-2 Induces a Durable and Antigen Specific Humoral Immunity after Asymptomatic to Mild COVID-19 Infection.” Edited by Etsuro Ito. PLOS ONE 17 (1): e0262169. https://doi.org/10.1371/journal.pone.0262169. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0262169.