Ever since the coronavirus disease 2019 (COVID-19) pandemic began, there have been many attempts to understand the nature and duration of immunity against the causative agent, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Permanent immunity is essential if the pandemic is to end. In the earlier SARS epidemic, antibodies were found to last for three or more years after infection in most patients. With the current virus, it may last for six or more months at least, as appears from some reports. Other researchers have concluded that immunity wanes rapidly over the same period, with some patients who were tested positive for antibodies becoming seronegative later on. This discrepancy may be traceable to variation in testing methods, sample sizes and testing time points, as well as disease severity.
Study details
The current study looked at a population of over a hundred convalescent COVID-19 patients, testing most of them for antibodies at five weeks and three months from symptom resolution.
The researchers used a multiplex assay that measured the Immunoglobulin G (IgG) levels against four SARS-CoV-2 antigens, one from SARS-CoV, and four from circulating seasonal coronaviruses. In addition, they carried out an inhibition assay against SARS-CoV-2 spike receptor-binding domain (RBD)-angiotensin-converting enzyme 2 (ACE2) binding and a neutralization assay against the virus. The antibody titers were then plotted against various clinical features and demographic factors.
Antibody titers higher in COVID-19 convalescents
The researchers found that severe disease is correlated with advanced age and the male sex. Patients with underlying vascular disease were more likely to be hospitalized with COVID-19, but those with asthma were relatively spared.
Convalescent COVID-19 patients had higher IgG levels against all four SARS-CoV-2 antigens, relative to controls, and in 98% of cases, at least one of the tests was likely to show higher binding compared to controls. IgGs targeting the viral spike and RBD were likely to be much more discriminatory between SARS-CoV-2 patients and controls. Interestingly, anti-SARS-CoV IgG, as well as anti-seasonal betacoronavirus antibodies, were likely to be higher in these patients.
Anti-spike and anti-nucleocapsid IgG levels, as well as neutralizing antibody titers, were higher in convalescent hospitalized COVID-19 patients than in convalescent non-hospitalized patients, and the titers were positively associated with disease severity.
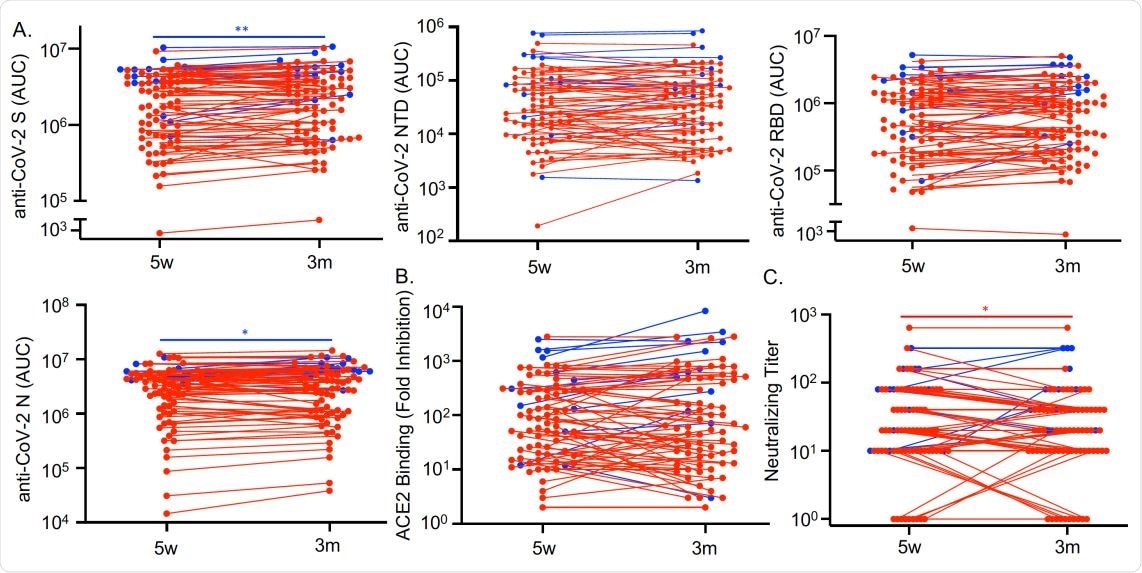
Antibodies against SARS-CoV-2 persist three months after COVID-19 symptom resolution. Sera from COVID-19 convalescent subjects (n=79) collected 5 weeks (w) and 3 months (m) after symptom resolution were subjected to multiplex assay to detect IgG that binds to SARS-CoV-2 S, NTD, RBD and N antigens (A), to RBD-ACE2 binding inhibition assay (B), and to SARS-CoV-2 neutralization assay (C). Dots, lines, and asterisks in red represent non-hospitalized (n=67) and in blue represent hospitalized (n=12) subjects with lines connecting the two time points for individual subjects (*p<0.05 and **p<0.01 by paired t test).
Clinical correlates of higher antibody titer
When antibody titers in non-hospitalized subjects were compared with clinical and demographic variables, they found that older males with a higher body mass index (BMI) and a Charlson Comorbidity Index score >2 were likely to have higher antibody titers. COVID-19 symptoms that correlated with higher antibody levels in these patients comprise fever, diarrhea, abdominal pain and loss of appetite. Chest tightening, headache and sore throat were associated with less severe symptoms.
The link between the specific symptoms listed above with higher antibody titers could indicate that they mark a robust systemic inflammatory response, which in turn is necessary for a strong antibody response. Diarrhea may mark severe disease, but it is strange that in this case, it was not more frequent in the hospitalized cohort. Alternatively, diarrhea may have strengthened the immune antibody response via the exposure of the virus to more immune cells via the damaged enteric mucosa. More study is required to clarify this finding.
Potential substitute for neutralizing assay
The binding assay showed that the convalescent serum at five weeks inhibited RBD-ACE2 binding much more powerfully than control serum. Neutralizing activity was also higher in these sera, but in 15% of cases, convalescent patients showed comparable neutralizing antibody titers to those in control sera. On the whole, however, there was a positive association between neutralizing antibody titer, anti-SARS-CoV-2 IgG titers, and inhibition of ACE2 binding.
Persistent immunity at three months
This study also shows that SARS-CoV-2 antibodies persist in these patients at even three months after symptoms subside, with persistent IgG titers against the SARS-CoV-2 spike, RBD, nucleocapsid and N-terminal domain antigens. Binding and neutralization assays remained highly inhibitory throughout this period. The same was true of antibodies against the other coronaviruses tested as well, an effect that has been seen with other viruses and could be the result of cross-reactive anti-SARS-CoV-2 antibodies. Alternatively, it could be due to the activation of memory B cells formed in response to infection by the seasonal beta-coronaviruses.
Conclusion
“IgG titers, particularly against S and RBD, and RBD-ACE2 binding inhibition better differentiate between COVID-19 convalescent and naive individuals than the neutralizing assay,” the researchers concluded.
These could be combined into a single diagnostic test, they suggest, with extreme sensitivity and specificity. The correlation with neutralizing antibody titers could indicate that the neutralizing assay, which is more expensive, sophisticated and expensive, as well as more dangerous for the investigators, could be replaced by the other antibody tests without loss of value.
In short, the study shows that specific antibodies persist for three months at least following recovery; antibody titers correlate with COVID-19-related fever, loss of appetite, abdominal pain and diarrhea; and are also higher in older males with more severe disease, a higher BMI and CCI above 2. Further research would help understand the lowest protective titer that prevents reinfection, and the duration of immunity.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Amjadi, M. F. et al. (2021). Fever, Diarrhea, and Severe Disease Correlate with High Persistent Antibody Levels against SARS-CoV-2. medRxiv preprint. doi: https://doi.org/10.1101/2021.01.05.21249240, https://www.medrxiv.org/content/10.1101/2021.01.05.21249240v1
- Peer reviewed and published scientific report.
Amjadi, Maya F., Sarah E. O’Connell, Tammy Armbrust, Aisha M. Mergaert, Sandeep R. Narpala, Peter J. Halfmann, S. Janna Bashar, et al. 2021. “Specific COVID-19 Symptoms Correlate with High Antibody Levels against SARS-CoV-2.” ImmunoHorizons 5 (6): 466–76. https://doi.org/10.4049/immunohorizons.2100022. https://journals.aai.org/immunohorizons/article/5/6/466/233992/Specific-COVID-19-Symptoms-Correlate-with-High.