Intramuscular (IM) vaccines can prevent severe disease and reduce the replication of the virus in the lower respiratory tract. However, even then, subgenomic and genomic viral RNA is detectable in samples from the nose, indicating that the virus can still infect the upper respiratory tract. Thus, local or mucosal immunity needs to be established to prevent transmission of the virus, and not just symptomatic disease.
Intranasal inoculation in first hamster study
The researchers immunized two groups of macaques with the vaccine, one group by the IM route and the other by the intranasal (IN) route. In both groups, high titers of binding IgG antibodies were high, as well as neutralizing antibodies. However, neutralizing antibody titers were higher in the IN group.
When challenged with a D614G SARS-CoV-2 isolate, the vaccinated animals did not lose weight. Both groups had daily swabs, and viral RNA was found in both groups, but at substantially lower titers in the IN group compared to the controls, from day 1 post-infection onwards. On the other hand, IM vaccination led to a reduction in viral RNA in swabs only on the seventh day post-infection. Total viral RNA shedding was also less in the IN group vs. the controls.
The amount of infectious virus shed in swabs, as well as the total amount of shedding, was lower in the IN group compared to the controls. The difference in viral RNA or infectious particle shedding was less significant in the IM group, relative to controls.
Necropsied animals in the IN group had no viral RNA or infectious virus in their lung tissue, while both were high in the control group. Two animals had weakly positive genomic RNA in the IM vaccinated group, but not infectious virus or subgenomic RNA. Pathologic lesions were found in the control group but not in the other two vaccinated groups.
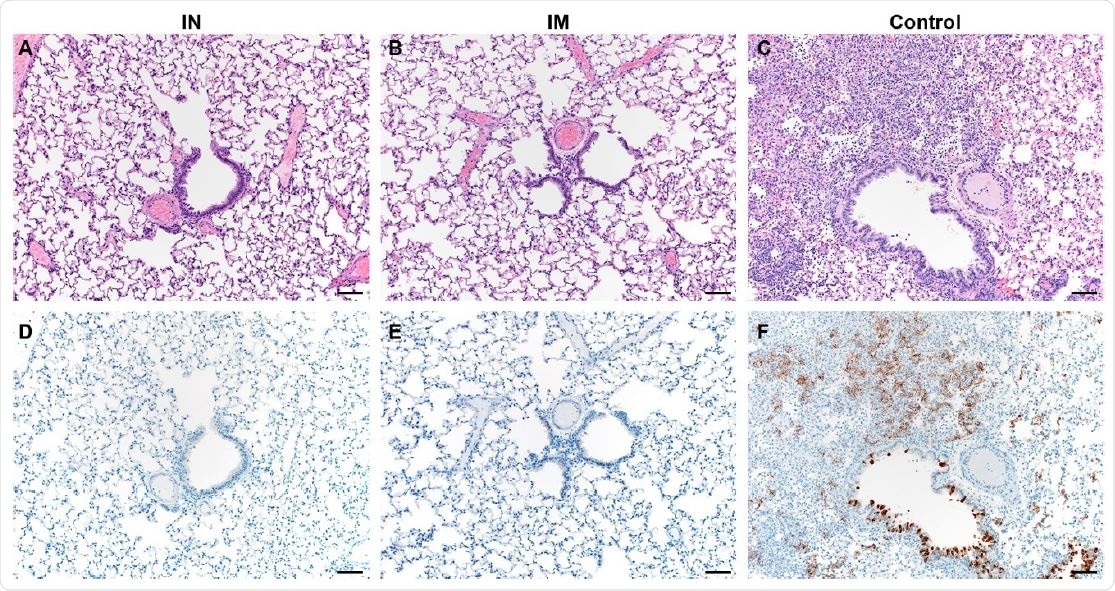
Pulmonary effects of direct intranasal challenge with SARS-CoV-2 in Syrian hamsters. A-C. H&E; D-F. IHC. A/B. No pathology. C. Moderate to marked interstitial pneumonia. D/E. No immunoreactivity. F. Numerous immunoreactive bronchiolar epithelial cells and Type I&II pneumocytes. Bar = 50μm.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Direct transmission hamster study
A direct hamster-to-hamster transmission model was also tested, using unvaccinated hamsters exposed to IN SARS-CoV-2 that were then allowed to live in the same cage with vaccinated animals for four hours, beginning 24 hours after exposure. The researchers found that vaccinated hamsters had high neutralizing antibody titers. Viral RNA and infectious virus shedding were present but lower in vaccinated animals, with a significant difference between controls and the IN group, but less so for the IM group.
Again, the total amount of shedding was significantly less for the IN group relative to the controls, for both viral RNA and infectious virus. This was not so with the IM group. There was no viral RNA or infectious virus in lung tissue in the IN group, but with the IM group, three animals had genomic RNA, and two subgenomic RNA, with the infectious virus being detectable in one animal’s lung tissue.
NHP studies confirm hamster findings
The next step was to test the results of IN vaccination in rhesus monkeys. They vaccinated four monkeys with two doses of IN vaccine. They found the ratio of IgA to total Ig to be higher in samples from the nose, compared to bronchoalveolar lavage fluid (BALF), and the serum. Anti-RBD and anti-S IgA antibodies were detected in serum and (weakly) in the nose at this point.
Moreover, IgG antibodies to the spike and receptor-binding domain (S and RBD, respectively) of the virus were detected in both serum and nasal samples, seven days after the first dose, but not in BALF. IgA specific antibodies were absent in BALF as well.
Systemic and local immunity
At the second time point, following the second dose, all samples had a higher IgG titer. Serum IgA antibodies to the S and RBD remained at the same level. However, IgA in the nose samples increased after the second dose and appeared in BALF as well.
Serum samples showed neutralizing antibodies at titers similar to that in human convalescent COVID-19 patients, and in NHPs following two doses of IM vaccine. Non-neutralizing Fc antibody-mediated effects were also observed, including complement deposition, phagocytosis and NK cell activation. Binding antibody titers against the RBD of the wild-type and one of the new SARS-CoV-2 variants were also found to be comparable.
When antibody profiles were examined, the researchers found significant differences between vaccinated and control animals as defined by local and systemic antibody development. They also found that low levels of nasal uptake of the virus correlated with low serum IgG, low neutralizing antibody titers, and significant viral shedding following exposure. High BAL IgG and IgA levels were found to be strongly correlated with low viral RNA in BAL and lung tissue, especially for subgenomic RNA relative to genomic RNA.
Heterologous protection
Thus, the study indicates that nasal shedding and viral load in the BAL are reduced in NHPs following IN vaccination, as in hamsters. This was observed despite the fact that the vaccine was based on the wild-type viral sequences, but the vaccinated animals were challenged using a different variant with a single change in the spike protein, namely D614G. This finding suggests that the vaccine protects against this variant as well. Probably, this can be extrapolated to other vaccines being developed. In fact, the researchers found the N501Y mutation-containing RBD to be equally bound by the antibodies induced by the vaccine used in this study.
What are the implications?
In conclusion, “IN vaccination resulted in systemic immunity comparable to that induced in vaccinated animals who received an IM vaccination, but also elicited SARS-CoV-2-specific mucosal immunity as demonstrated by IgA detection in nasosorption and BAL samples.”
Secondly, in the direct transmission hamster study, IN vaccination completely protected the lower respiratory tract, but partial protection was achieved in the IM group. This could be by seeding of the virus from the upper respiratory tract in the IM group because of increased viral shedding from the nose in this group. This was not observed in the first hamster study, where the virus was directly inoculated into the nasal cavity in both groups.
Another explanation could be the difference in the site of initial infection, as direct transmission might result in the virus reaching the lung almost immediately, but not with an inoculum of 40 μl, as used in this study, in the first hamster group. This is rendered more probable by the fivefold rise in infectious virus titer in the lungs of controls in the second hamster study relative to the first.
“The data presented here demonstrate SARS-CoV-2 specific mucosal immunity is possible after IN vaccination, and results in a reduction in nasal shedding. It is now pertinent to investigate whether this finding translates to the clinic.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources