A recent study by US researchers shows how the 501Y.V2 variant of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), characterized by several mutations, is able to escape neutralization by present first-wave anti-SARS-CoV-2 antibodies and potentially re-infect COVID-19 convalescent individuals. The paper is currently available on the bioRxiv* preprint server.
As many variants of SARS-CoV-2 emerge and subsequently displace first-wave viruses, it is pivotal not only to appraise their relative transmissibility and virulence in causing coronavirus disease (COVID-19), but also their propensity to escape antibody neutralization.
Of utmost interest are variants harboring mutations that can affect the interaction of the viral spike receptor-binding domain (S RBD) with the viral receptor on host cells, angiotensin-converting enzyme 2 (ACE2), which provides an entry point for the coronavirus.
Variants with a greater binding affinity for ACE2 are likely to spread more. Furthermore, transmissibility is linked to mortality, as an inevitable increase in infection rates caused by the novel variants will result in higher disease and death toll.
However, these dire repercussions of more rapid and widespread infections can also be compounded by a loss of efficacy of currently available antibody-based treatments and vaccines and a decrease of protective immunity in individuals previously infected with a 'first wave' virus.
In order to improve our understanding of the risks posed by an individual or combined mutations in these 'second wave' variants, a research group from ImmunityBio company in California conducted a computational analysis of interactions of the S RBD with human ACE2.
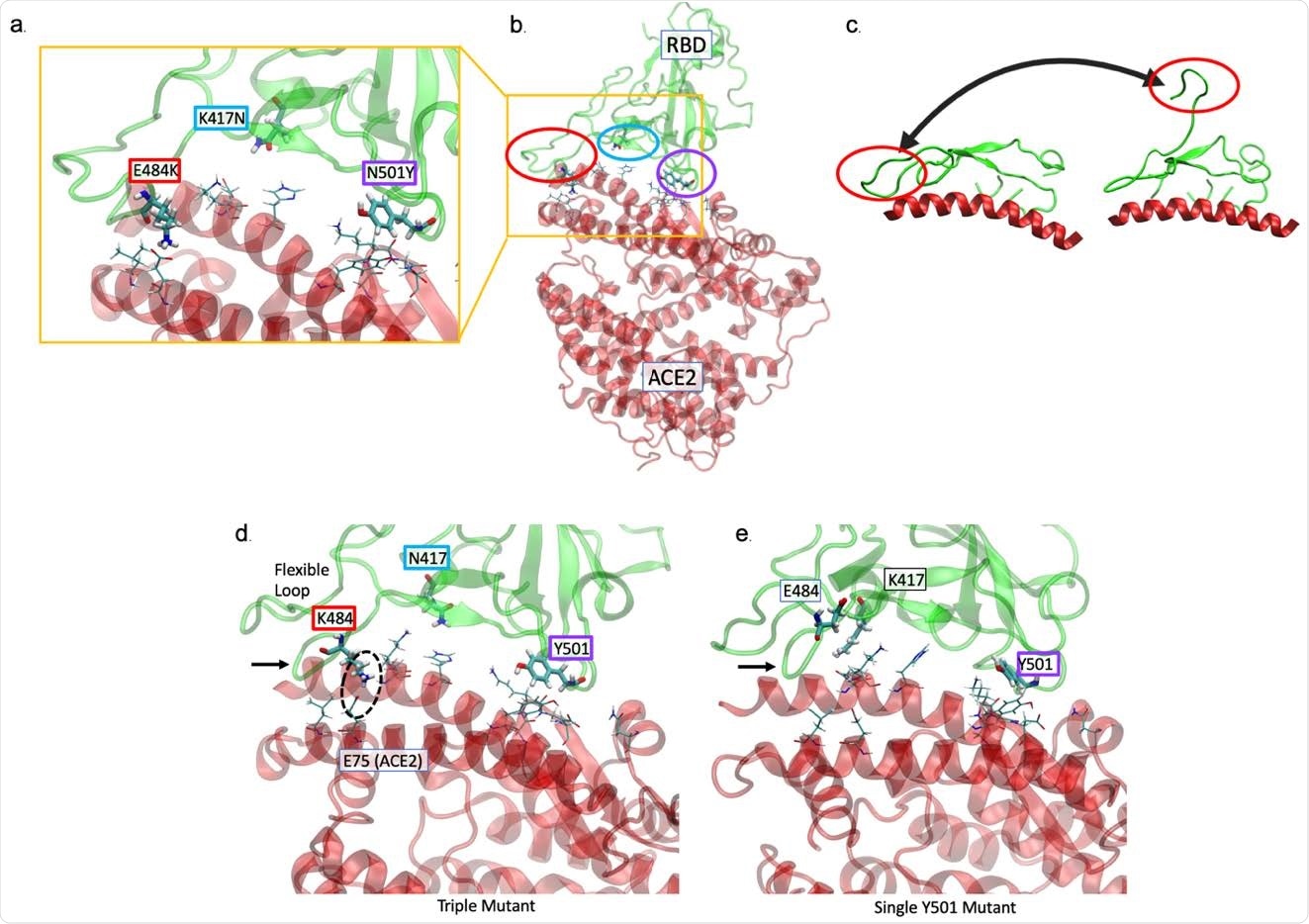
The K484 substitution in the novel South African variant increases affinity of the spike receptor-binding domain (S RBD) for ACE2. (a, b) The positions of the E484K (red), K417N (cyan), and N501K (purple) substitutions at the interface of the 501Y.V2 variant S RBD - hACE2 interface are shown. hACE2 residues nearest to the mutated RBD residues are rendered as thin sticks. The E484K mutation is located in a highly flexible loop region of the interface, K417N in a region with lower probability of contact, and N501K at a second point of high-affinity contact. (c) The range of movement available to the loop containing residue 484 is shown by PCA of MD simulation of a first-wave sequence11,13. (d) MD simulation performed in the presence of all 3 substitutions reveals the loop region is tightly associated (black arrow) with hACE2. A key contact ion pair is circled. (e) In comparison to K484, when E484 (‘wildtype’) is present with only the Y501 variant, the loop is not as tightly associated (arrow).
In silico simulation methods
In this study, the researchers have utilized millisecond-scale MD simulation methods to investigate mutations (E484K, K417N, and N501Y) at the S RBD-ACE2 interface in the rapidly spreading South African variant 501Y.V2 – and their effects on RBD binding affinity and spike glycoprotein conformation.
The wild-type ACE2/RBD complex was built from the cryo-electron microscopy structure. Moreover, ten copies of each RBD mutant were minimized, equilibrated and simulated, and the minimization processed occurred in two phases.
Finally, principal component analysis (PCA) was pursued by using the full set of simulations of the triple mutant, E484K and N501Y systems. Simulation structures were extrapolated onto the eigenvectors for every mutation system.
The great escape from neutralization
The study revealed greater affinity of K484 S RBD for ACE2 in comparison to E484, as well as the greater probability of modified conformation when compared to the original structure. This may actually represent mechanisms by which the new 501Y.V2 viral variant was able to replace original SARS-CoV-2 strains.
More specifically, both E484K and N501Y mutations were shown an increase affinity of S RBD for human ACE2 receptor, while E484K was able to switch the charge on the flexible loop region of RBD, resulting in the formation of novel favorable contacts.
The aforementioned improved affinity is a likely culprit for more rapid spread of this variant due to greater transmissibility, which is a prime reason why it is important to track these mutations and act in a timely manner.
Furthermore, the induction of conformational changes is responsible for the escape of the 501Y.V2 variant (distinguished from the B.1.1.7 UK variant by the presence of E484K mutation) from neutralization by existing anti-SARS-CoV-2 antibodies and re-infect COVID-19 convalescent individuals.
Implications for further vaccine design
"We believe the MD simulation approach used here similarly represents a tool to be used in the arsenal against the continuing pandemic, as it provides insight into the likelihood mutations alone or in combination may have effects that lessen the efficacy of existing therapies or vaccines", say the authors of this study.
"We suggest vaccines whose efficacies are largely dependent upon humoral responses to the S antigen only are inherently limited by the emergence of novel strains and dependent upon frequent re-design," they add.
On the other hand, a vaccine that evokes a vigorous T-cell response is much less subject to changes due to accruing mutations and, thus, provides a better and more efficient approach to protection against this disease.
Finally, the ideal vaccine would also incorporate a second, conserved antigen (such as the SARS-CoV-2 nucleocapsid protein), which would likely elicit an effective humoral and cell-mediated immune response - even when confronted with a rapidly changing virus.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.