The coronavirus disease 2019 (COVID-19) pandemic has caused over 2.15 million deaths, among over a hundred million documented cases of infection with the causative agent, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). About eight in ten deaths have occurred in older people, who make up only a tenth of the total cases.
The gold standard for the diagnosis of SARS-CoV-2 infection is by reverse transcriptase-polymerase chain reaction (RT PCR) testing, using nasopharyngeal (NP) swabs, oropharyngeal (OP) swabs, nasal swabs, or saliva, usually within five days of exposure. In most cases, symptoms begin within five days of exposure.
The current study evaluates the use of antibody testing to detect exposure to the infection, identify potential immunity, and find cases within a household where there has been a documented exposure or infection.
In PCR-positive individuals, Immunoglobulin M (IgM) antibodies appear within a median of five days from the earliest symptom and wane with time. Immunoglobulin G (IgG) and neutralizing antibodies appear in a median of 14 days following symptom onset and persist for months.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Antibody levels increase with age
The study used IgG values from samples obtained from over 79,000 subjects and IgM levels from about 62,000 of them. About 5% and 3% were positive for IgG and IgM, respectively, with median IgG titers of ~4 AU/mL, ~10 AU/mL, and ~11 AU/mL in individuals aged less than 45 years, 45-64, and 65 years or more, respectively. This shows that titers are more than double in the two older age strata relative to the youngest, for both males and females. However, males had higher IgG levels than females in the 45-64-year age group.
Secondly, IgM titers were more likely to be detectable in females above 65 years than in the youngest age group.
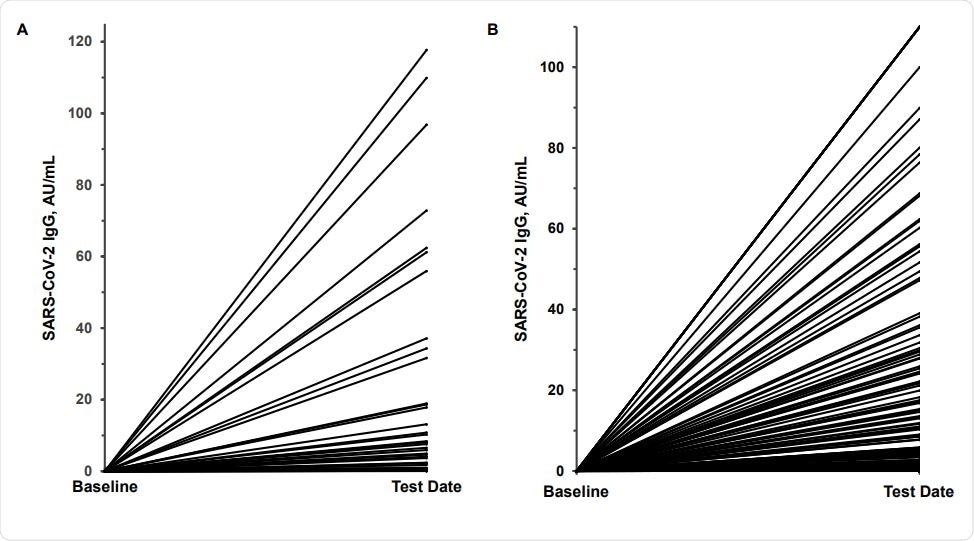
Variability in SARS-CoV-2 IgG antibody response. Panel A shows the response in 41 meatpacking plant employees who were SARS-CoV-2 RNA positive 2 weeks prior to testing; panel B, the response in 129 potential convalescent plasma donors 4-6 weeks after a SARS-CoV-2 RNA positive test. The baseline value was assumed to be IgG 0.05 AU/mL.
Antibodies in meat-packing plant employees
Of the ~350 meat-packing plant employees tested, IgG positivity occurred in almost a fifth (19%), and IgM positivity in ~15%. At another plant, of 217 employees tested, a quarter (51 individuals) were PCR positive. Most (75%) of the 41 PCR-positives retested two weeks later continued to be positive.
At this point, ~71% and ~10% showed seroconversion for IgG and IgM, respectively. Almost two in three reported symptomatic infection. Median IgG and IgM values in all the 41 patients tested at two weeks from infection were 20.5 and 0.5 AU/mL, respectively, but the IgG titers varied over an extensive range.
Interestingly, 25 PCR-negative individuals who requested antibody tests due to their symptoms and a history of exposure had positive IgG and IgM levels in 64% and 28%, respectively. All those who were IgM positive had persistent symptoms. Median titers for IgM and IgG in these individuals were ~25 and 1 AU/mL, respectively.
“These data clearly document the benefits of antibody testing for case finding in previously exposed subjects even with negative RNA tests.”
Antibodies in healthcare provider’s office settings
In one healthcare provider’s office in the Bronx, New York, almost 290 patients were tested for antibodies. About 18% and 5% had positive and borderline IgG values. Symptoms were reported in six of ten patients in the borderline group, with three having a history of PCR positivity, and six having been exposed to the virus.
Antibodies in family clusters
The researchers found that when PCR and antibody positivity were compared in 154 outpatients, 22 individuals (~14%) were positive for both. Seven of these were followed up, with their families, and nine solitary positive individuals, many with suggestive symptoms. Three of the individuals followed up, all older than 80 years, required hospital admission, two were placed on ventilation, and one died.
The data indicated that antibody testing could identify additional cases in family clusters, that PCR positivity could persist for up to six weeks, and IgM levels may be increased for prolonged periods in patients with persistent symptomatic illness.
Antibodies and inflammatory molecules in PCR positives
The researchers also compared antibodies (IgM, IgG, and neutralizing antibodies) and specific inflammatory markers (IL-6, hsCRP, and ferritin) between PCR negatives and two groups of PCR positives (outpatients and inpatients). They found that all controls were antibody negative.
PCR positives had 300-fold and 600-fold higher median IgG titers in outpatients and inpatients, respectively, relative to controls. Again, there was a wide variation between individual patients (1-200 AU/mL and 0.05 to 170 AU/mL, in outpatients and inpatients, respectively.
IgM values also showed two-fold and five-fold higher values in outpatients and inpatients compared to controls. Neutralizing antibodies also had 12-fold and 24-fold higher median levels in outpatients and inpatients, respectively. There were marked inter-individual variations in both these cohorts from about 1-14 AU/mL in outpatients and 0.5-19 AU/mL in inpatients, respectively.
The data shows substantially higher antibody titers in PCR positives than controls.
With IL-6, median titers in controls did not vary from that in outpatients, but inpatients had 75 times higher median titers compared to other groups. However, the range of individual titers extended from 0.75-5500 pg/mL. The same pattern was seen with hs-CRP, with 80-fold higher median titers, and ferritin with nine-fold higher median titers.
If the hs-CRP cut-off was 10 mg/L or more, 93% of inpatients, but only 6% of controls or outpatients, would have positive tests. The researchers suggest that being PCR-positive, with hs-CRP values above this threshold, were at 17 times the risk of requiring hospitalization than if the hs-CRP values were below this threshold, making it a useful predictor of disease severity.
Algorithm for hospitalization risk
The researchers developed a new algorithm to predict patients who would require hospitalization. They used IL-6, hs-CRP, and IgM as markers, all being part of the ‘cytokine storm’ associated with severe COVID-19, with thresholds of ≥10 pg/mL ≥10 mg/L, and ≥1.0 AU/mL, respectively. They found that elevations in these molecules predicted hospitalization in ~86%, ~60%, and ~12% of patients.
If two or more of these were elevated, almost 98% would be hospitalized, but only 2% of the others, indicating a 50-fold risk in this group. IgG, neutralizing antibody, and ferritin was not included as they did not increase the value of the model further.
What are the implications?
The study shows a twofold antibody titer in older patients. Moreover, they found that antibody testing is helpful in case identification, as well as to monitor antibody levels in convalescent plasma (CP).
The researchers also found significantly high variability in antibody titers in PCR-positive patients. If the IgG threshold is set above >6.5 AU/mL (1:320, as per FDA guidelines), only half the PCR positive patients would be positive. A third would have levels >20 AU/mL (>1:1000).
IgG levels closely associate with neutralizing antibody levels. And finally, when two or more of the markers IL-6 ≥10 pg/mL, hs-CRP ≥10.0 mg/L, and/or IgM ≥1.0 AU/mL are present, the patient is very likely to require hospitalization.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Schaefer, E. J. et al. (2021). Clinical utility of Corona Virus Disease-19 serum IgG, IgM, and neutralizing antibodies and inflammatory markers. medRxiv preprint. doi: https://doi.org/10.1101/2021.01.19.21249604. https://www.medrxiv.org/content/10.1101/2021.01.19.21249604v1
- Peer reviewed and published scientific report.
Schaefer, Ernst J., Latha Dulipsingh, Florence Comite, Jessica Jimison, Martin M. Grajower, Nathan E. Lebowitz, Maxine Lang, et al. 2021. “Corona Virus Disease-19 Serology, Inflammatory Markers, Hospitalizations, Case Finding, and Aging.” PloS One 16 (6): e0252818. https://doi.org/10.1371/journal.pone.0252818. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0252818.