Given the unchecked resurgence of the coronavirus disease 2019 (COVID-19) pandemic, the need of the hour is an effective vaccine. While over 237 vaccines are in clinical development or pre-clinical development, several have received emergency use authorization and are being rolled out in various countries.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Study details
This is the first human study of this candidate vaccine from Sanofi Pasteur in collaboration with GlaxoSmithKline.
The study was conducted as a phase I/II double-blind study in 439 healthy adults in the USA. They were randomly assigned to receive either placebo or the candidate vaccine as either a single-dose or two-dose regimen. The doses used were either low-dose or high-dose adjuvanted antigen or high dose antigen without adjuvant. The adjuvant was either AF03 or AS03. Both AS03 and AF03 are oil-in-water emulsions.
There were 269 participants aged 18-49 years who received two doses, and 170 who got a single dose. There were 90 and 50 aged 50 years or above who received two doses and one dose, respectively.
The participants were followed up for 43 days. The researchers measured neutralizing and binding antibodies in serum on days 1, 22 and 36.
The vaccine
The spike protein on the viral surface mediates host cell entry. It can be stabilized in the prefusion conformation by introducing a double proline substitution before the S2 subunit's central helix. This elicits robust neutralizing responses in mice.
This study's candidate vaccine was the Sanofi Pasteur SARS-CoV-2 recombinant protein CoV2 preS dTM vaccine containing the stabilized SARS-CoV-2 prefusion S protein. Recombinant protein vaccines are potentially cheaper and lower in cost relative to live attenuated or inactivated vaccines. They can be adjuvanted to increase the potency and quality of the immune response. The use of an adjuvant also allows less vaccine antigen to be used for the same robust antibody response. This could be a critical advantage when the supply of antigen is potentially limited.
The adjuvant
The researchers found that there were no immediate or serious adverse effects (AEs), severe AEs that required medical attention, or any other particular AE.
Safety
Grade 3 solicited reactions were more significant in frequency than expected after the second dose in both the groups that received the adjuvanted vaccines. These included pain, redness and swelling at the injection site, and systemic reactions like fever, headache, malaise, and myalgia. The adjuvant-free high-dose formulation had a lower frequency of these reactions, comparable to placebo.
The groups that received the AS03-adjuvanted versus AF03- adjuvanted vaccine, with high doses relative to low doses; and in younger patients compared to older patients. also had less common and less severe solicited AEs. No grade 3 reactions at the injection site occurred after the first dose or after the adjuvant-free high dose administration.
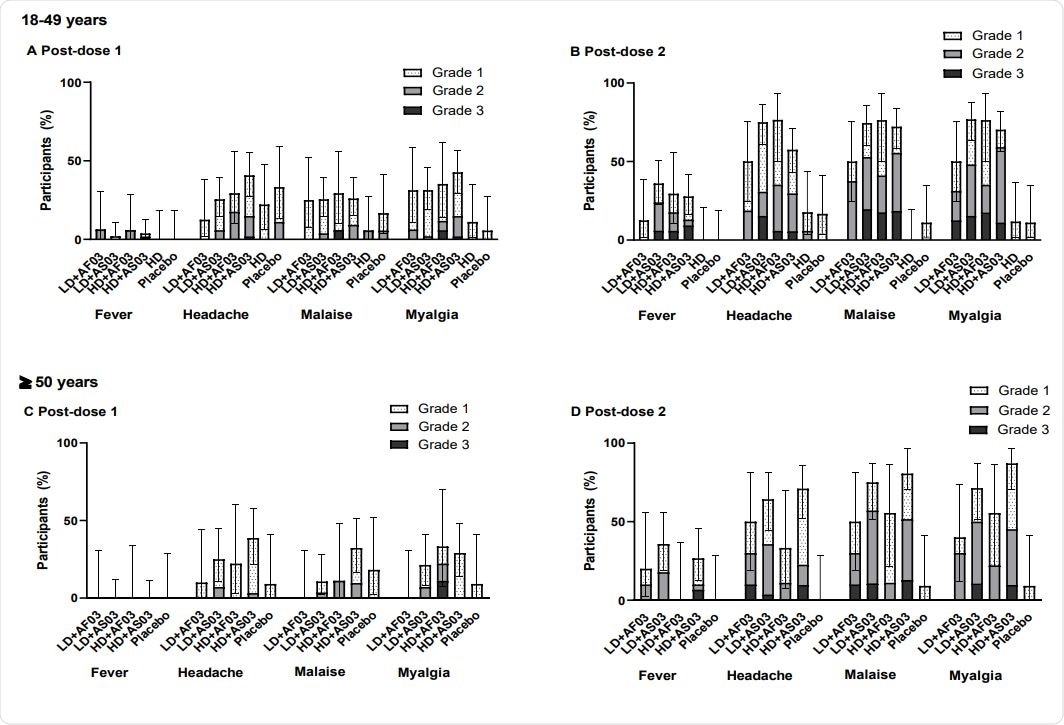
The frequency of solicited systemic reactions after the first or second dose in participants from the two-dose cohort (SafAS)
Immunogenicity
A single dose of any vaccine did not effectively induce neutralizing antibodies, indicating that two doses are necessary for immunization.
Adjuvanted groups had higher neutralizing and binding antibody titers relative to the groups that received unadjuvanted vaccines. The titers of these antibodies were higher in groups that received the AS03-adjuvanted versus AF03- adjuvanted vaccine, with high doses relative to low doses; and in younger patients compared to older patients.
Adjuvanted high-dose regimens elicited three- and four-fold higher titers than low doses, for AF03 and AS03, respectively, at day 36. Seroconversion occurred in 88% of those who received adjuvanted high doses relative to 52% with low-dose adjuvanted regimens.
Participants aged 50 years or more had lower neutralizing antibody titers, indicating an age-dependent efficacy with these formulations. In the subset aged 60 years or upwards, who are at high risk following infection with SARS-CoV-2, very low seroconversion rates were observed. Even with high-dose adjuvanted regimens, only ~63% of the latter group seroconverted.
The researchers comment, "We, therefore, conclude that the vaccine candidates tested here have not adequately evaluated the antigen formulation and dose needed to ensure optimal immune responses, including in those most at risk."
Cell-mediated immunity
Cell-mediated immunity (CMI) was measured in some participants from both cohorts. There were more significant increases in IFN-γ, IL-2 and TNFα cytokines than for IL-4, IL-5 and IL-13, indicating a Th1-favoring response.
What are the implications?
More work is required to optimize the antigen formulation and purification process since the antigen's actual dose used in the current study had to be recalculated after one dose, revealing an error in the previous protocol. As a result, the host cell protein was higher than planned, and the protein antigen concentration lower, correspondingly. The host cell protein content should be reduced to prevent adverse reactions.
There were no signs of vaccine-mediated antibody-dependent enhancement (ADE) following exposure to the wild-type virus.
The lower immune response, especially with the older patients, is likely due to the lower dose of antigen in the vaccine dose than originally planned.
The higher antigenicity after dose 2 is probably due to the excessive presence of protein derived from the host cells. Thus, further work is necessary to determine the optimal formulation, including the antigen dose and adjuvant type. Encouragingly, no serious safety issues were noted, allowing further development of this vaccine.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Source:
Journal references:
- Preliminary scientific report.
Goepfert, P. A. et al. (2021). Safety and immunogenicity of SARS-CoV-2 recombinant protein vaccine formulations in healthy adults: a randomised, placebo-controlled, dose-ranging study. medRxiv preprint. doi: https://doi.org/10.1101/2021.01.19.20248611. https://www.medrxiv.org/content/10.1101/2021.01.19.20248611v1
- Peer reviewed and published scientific report.
Goepfert, Paul A., Bo Fu, Anne-Laure Chabanon, Matthew I. Bonaparte, Matthew G. Davis, Brandon J. Essink, Ian Frank, et al. 2021. “Safety and Immunogenicity of SARS-CoV-2 Recombinant Protein Vaccine Formulations in Healthy Adults: Interim Results of a Randomised, Placebo-Controlled, Phase 1–2, Dose-Ranging Study.” The Lancet Infectious Diseases 0 (0). https://doi.org/10.1016/S1473-3099(21)00147-X. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00147-X/fulltext.