The worldwide increase in the circulation of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to the crossing of the shocking milestone of 100 million reported cases. Among the chief causes of growth in infection numbers have been the emergence of new variants, notably the UK variant, with increased infectivity relative to the ancestral strain. However, a new preprint research paper, posted to the preprint server bioRxiv*, presents data indicating that the Indian-made Covaxin vaccine candidate successfully neutralizes this variant, preventing infection when challenged by it.
The UK variant is also called the Variant of Concern (VOC) 202012/01, also known as lineage B.1.1.7 or 20B/501Y.V1. It has rapidly increased as a proportion of new cases of COVID-19 in the UK in the space of fewer than two months. B.1.1.7 has been sequenced from other countries as well, and since direct flights connected many such affected regions to and from the UK, the potential for a dramatic rise in cases worldwide is obvious.
Similar concerns have been expressed about the so-called South African variant, underlining the issues raised by these variants concerning the newly available COVID-19 vaccines' protection.
The UK variant has 17 mutations in its genome, with eight on the spike receptor-binding domain (RBD), which allows the virus to attach to the angiotensin-converting enzyme 2 (ACE2) receptor on the target host cell.
The UK has rolled out vaccination with the Pfizer-BioNTech vaccine, and the Oxford Astra-Zeneca vaccine. Both are mRNA vaccines, inducing spike antigen expression of earlier SARS-CoV-2 strains. The million-dollar question is whether the many spike mutations found in the later variants will affect the ability of vaccine-induced antibodies directed against the earlier D614G spike version to neutralize the virus.
The vaccine
Covaxin is an indigenous inactivated whole-virion SARS-CoV-2 vaccine BBV152. It has been reported to have high neutralization potency in a phase I clinical trial against the homologous hCoV-19/India/2020770 strain, as well as two other heterologous unclassified cluster strains, hCoV-19/India/2020Q111 and hCoV-19/India/2020Q100. Notably, all three strains possess the N501Y mutation characteristic of the UK strain spike RBD.
Phase II trials showed impressive results with the plaque reduction neutralization test (PRNT50), with immune serum from Covaxin recipients showing the ability to reduce the level of viral infection of cells in culture at low titers following immunization. Seroconversion and neutralizing antibody production occurred in 99.6% of subjects.
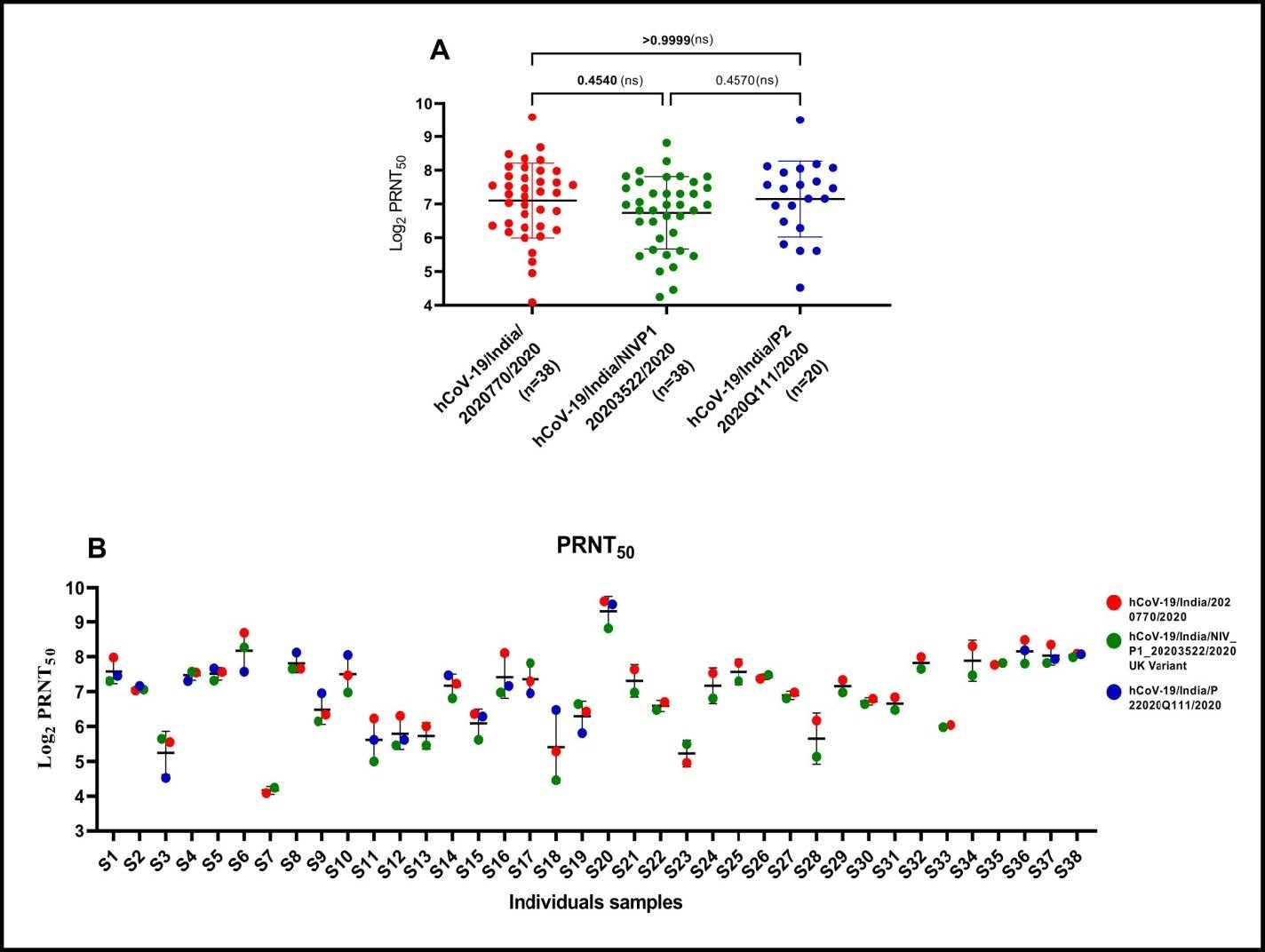
(A) Neutralising antibody response of BBV152/COVAXINTM vaccinated sera against SARS CoV-2 strains: Neutralizing antibody titers (PRNT50) of vaccine’s sera against hCoV-19/India/2020770 (homologous), hCoV-19/India/20203522 (heterologous UK strain) and hCoV-19/India/2020Q111 (heterologous unclassified cluster). The bar represents the geometric mean and standard deviation of the respective group titers. Non-parametric Kruskal-Wallis test was used for the comparison of the PRNT50 values from different groups. The p-values above 0•05 were considered non-significant and are marked on the figure (ns-non-significant). (B) Comparison of PRNT50 value of each vaccine recipient’s sera with three strains of SARS CoV-2: Neutralizing antibody titers (PRNT50) of each vaccinees’ sera against hCoV- 19/India/2020770 (homologous), hCoV-19/India/20203522 (heterologous UK strain) and hCoV- 19/India/2020Q111 (heterologous unclassified cluster).The bar represents the mean and standard deviation of the respective sera.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Study shows efficacy against UK variant
The current study reports the results of neutralization testing of serum samples obtained from phase II trial participants against the UK variant.
The researchers first isolated and sequenced the UK variant from UK-returnees in India. The isolate hCoV-19/India/20203522 displayed all the characteristic mutations found in SARS-CoV-2 (VOC) 202012/01.
The sera from 38 BBV152-immunized subjects were tested against the UK variant. Simultaneously, sera from 20 subjects were tested against one of the heterologous unclassified strains listed above, hCoV-19/India/2020Q111.
The PRNT50 tests showed a median ratio of 0.8 and 0.9 neutralization efficacy when the homologous strain hCoV-19/India/2020770 was compared with the UK variant hCoV19/India/20203522 and the other heterologous strain hCoV-19/India/2020Q111, respectively.
In other words, the neutralizing antibodies elicited by immunization with BBV152/Covaxin were similarly effective against both the locally circulating homologous strain hCoV-19/India/2020770 and the UK variant hCoV-19/India/20203522. It also proved effective in neutralizing another heterologous strain, hCoV-19/India/2020Q111.
What are the implications?
Earlier studies have shown that the UK variant evaded neutralization with convalescent plasma containing high titers of neutralizing antibodies. This was demonstrated to be because of the presence of the E484 substitution along with a subsequent 11-amino-acid insertion in the NTD N5 loop of the spike antigen.
This led to a great deal of uncertainty as to whether the current vaccines would neutralize the new variants. The current study alleviates such concerns by showing highly comparable neutralization activity of vaccinated individuals against the virus's homologous and heterologous strains. These results indicate that the mutations in the UK variant do not mediate immune escape to neutralizing antibodies elicited by Covaxin vaccination.
Covaxin has been rolled out in India's vaccination program, the largest in the world, along with the Indian-manufactured Oxford Astra-Zeneca vaccine, distributed under the name Covishield. The researchers say, "It is unlikely that the mutation 501Y would be able to dampen the potential benefits of the vaccine."

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.