Researchers in the United States have shown that two doses of vaccine may not be necessary to protect against coronavirus disease 2019 (COVID-19) among people who have already been infected with the causative agent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The study of more than 100 people found that antibody responses to one vaccine dose among individuals with pre-existing immunity exceeded responses among naïve individuals who received two vaccine doses.
The team from Icahn School of Medicine at Mount Sinai in New York also found that vaccine reactogenicity following one dose was much more common among those who had already been infected with SARS-CoV-2.
“Changing the policy to give these individuals only one dose of vaccine would not negatively impact on their antibody titers, would spare them from unnecessary pain and free up many urgently needed vaccine doses,” says Professor of Vaccinology Florian Krammer and the team.
Professor Krammer (@florian_krammer) is a member of the Vaccine and Edward Jenner Society Young Investigator Program and a scientific adviser for enGenes and PathSensors. The Krammer laboratory is also currently working with Pfizer on animal models of the SARS-CoV-2 virus.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The situation so far
In December 2020, the US Food and Drug Administration (FDA) authorized the emergency use of two vaccines against SARS-CoV-2 – the Pfizer/BioNTech BNT162b2 vaccine and Moderna’s mRNA-1273 vaccine.
Findings from Phase 3 trials showed that in the case of each vaccine, two doses administered 3 to 4 weeks apart prevented symptomatic SARS-CoV-2 infection among participants who had not previously had COVID-19.
“For individuals with pre-existing immunity to SARS-CoV-2, the first vaccine dose likely immunologically resembles the booster dose in naïve individuals,” writes Krammer and colleagues. “Anecdotally, individuals with pre-existing immunity also experience more severe reactogenicity after the first dose, compared to naïve individuals,” they add.
The team says this begs the question of whether individuals with pre-existing immunity should even receive a second vaccine dose.
What did the researchers do?
The researchers assessed antibody responses among 109 individuals with (n=41) and without (n=68) established SARS-CoV-2 immunity who received one dose of vaccine in 2020 and another dose 3 to 4 weeks later.
The antibody titers generated in response to the first vaccine among individuals with immunity were equal to or even higher than among naïve individuals who received a second dose.
Repeated sampling (at 9-12 days, 13-16 days, 17-20 days and 21-24 days) following the first vaccine found that most naïve individuals launched variable and low anti-SARS-CoV-2 immunoglobulin G (IgG) responses within 9 to 12 days.
By contrast, individuals with pre-existing immunity quickly generated uniform high levels of anti-SARS-CoV-2 IgG antibodies within days of vaccination.
Antibody titers were between 10 and 20 times higher among those with immunity across all timepoints, and were still more than ten times higher even when naïve individuals had received a second dose.
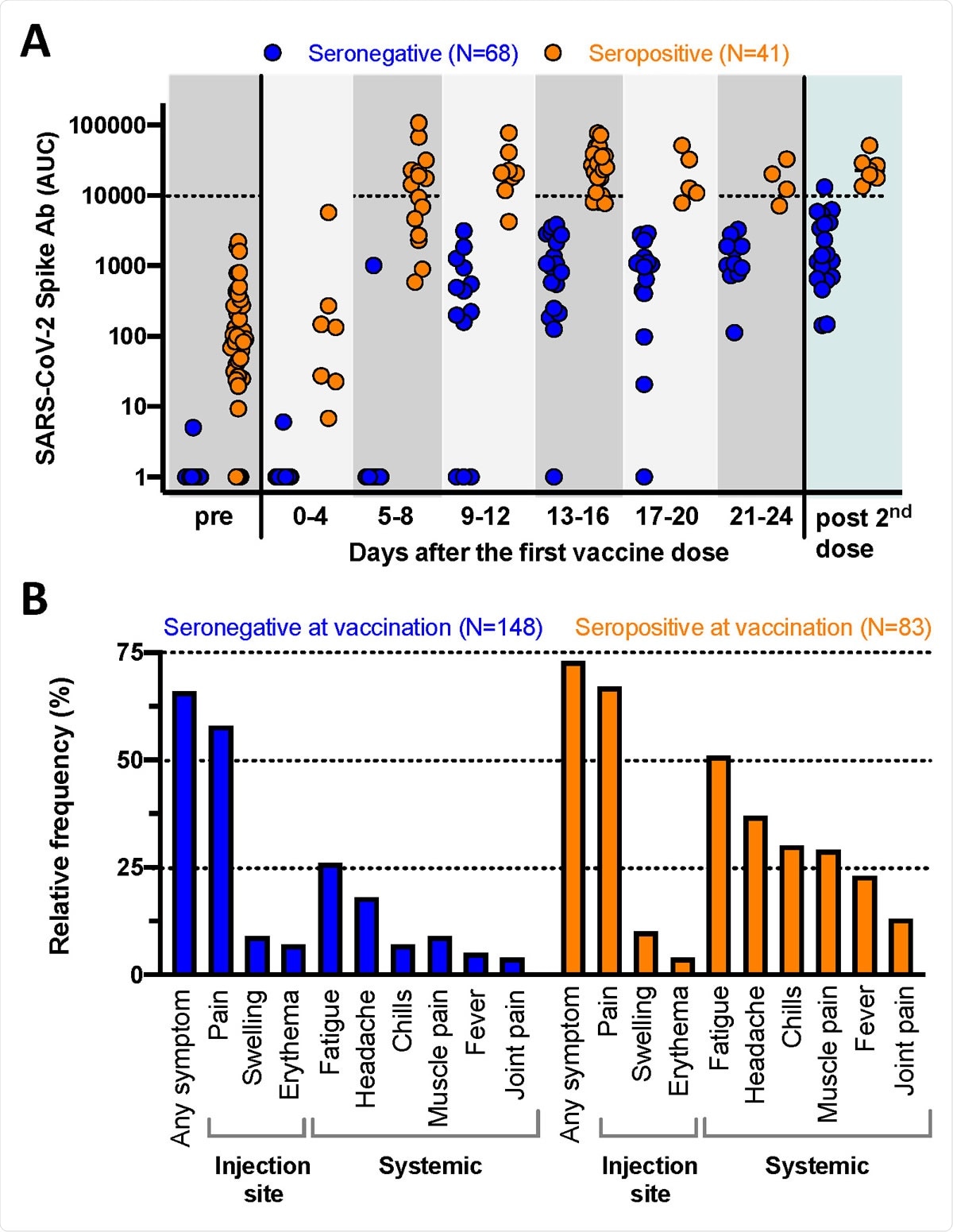
Immunogenicity and reactogenicity of SARS-CoV-2 RNA vaccines. A: Quantitative SARS-CoV-2 spike antibody titers (ELISA, expressed as area under the curve, AUC) for 109 individuals. “Pre” represents the antibody response prior to vaccination while “post 2nd dose” indicates the immune responses mounted after the second vaccine dose. Note that some of the individuals with pre-existing immunity had antibody titers below detection (AUC of 1) at the time point prior to vaccination. B: Vaccine associated side effects experienced after the first dose (N= 231 individuals). The local side effects occur with comparable frequency while the systemic symptoms are significantly more common in the individuals with pre-existing immunity.
Reactogenicity was also much more pronounced in people with immunity
The team also found that reactogenicity following one vaccine dose was substantially more pronounced among those with pre-existing immunity. These individuals reported similar side-effects to those reported by individuals who received two doses in phase 3 trials.
The most common localized reactions were pain, swelling and erythema, which occurred at the same frequency between the two groups.
However, systemic side effects such as fever, headache, muscle or joint pain were significantly more frequent among those with pre-existing immunity than among naïve vaccine recipients.
What are the implications of the study?
The researchers say the findings suggest that a single vaccine dose elicits very rapid immune responses among individuals with existing immunity and generates equal or even higher levels of antibody titers than among naïve individuals who received two doses.
“These observations are in line with the first vaccine dose serving as a boost in naturally infected individuals providing a rationale for updating vaccine recommendations to considering a single vaccine dose to be sufficient to reach immunity,” writes Krammer and colleagues.
“Such policies would allow not only expanding limited vaccine supply but also limit the reactogenicity experienced by COVID-19 survivors,” concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Krammer F, et al. Robust spike antibody responses and increased reactogenicity in seropositive individuals after a single dose of SARS-CoV-2 mRNA vaccine. medRxiv, 2020. doi: https://doi.org/10.1101/2021.01.29.21250653, https://www.medrxiv.org/content/10.1101/2021.01.29.21250653v1
- Peer reviewed and published scientific report.
Krammer, Florian, Komal Srivastava, Hala Alshammary, Angela A. Amoako, Mahmoud H. Awawda, Katherine F. Beach, Maria C. Bermúdez-González, et al. 2021. “Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 MRNA Vaccine.” New England Journal of Medicine, March. https://doi.org/10.1056/nejmc2101667. https://www.nejm.org/doi/10.1056/NEJMc2101667.