As the need for rapid diagnosis of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) increases, due to the relentless surge in coronavirus disease 2019 (COVID-19) cases worldwide, new rapid antigen tests that offer higher sensitivity are being developed. A new preprint posted to the medRxiv* server describes the use of silver amplification technology to develop a sensitive, rapid antigen test for COVID-19.
The only methods to contain the current pandemic are non-pharmaceutical, given that there are no specific and effective antivirals and that the first vaccines are only now being administered. These methods depend on early diagnosis of infection, preferably before they become infectious so that they can be isolated along with their close contacts.
The gold standard diagnostic test for SARS-CoV-2 at present, in terms of sensitivity and reliability, is the reverse transcription-quantitative polymerase chain reaction (RTqPCR), a nucleic acid amplification test (NAAT). The disadvantages of this approach in containing the transmission of the virus are obvious. It requires a number of technical processes such as viral RNA extraction from the clinical specimens and sophisticated equipment and facilities, available only in centralized laboratories. This is bound to increase the time required for testing and convey the results and make widespread testing difficult in low-resource and remote settings.
Rapid diagnostic tests
The need of the hour is a SARS-CoV-2 antigen rapid diagnostic test (Ag-RDT). This, if found to be sufficiently sensitive, could be developed to be a point-of-care test (POCT). This would allow results to be obtained within 30 minutes of taking the sample, without either special training or equipment.
The majority of currently available SARS-CoV-2 Ag-RDT testing kits use the antigen capture sandwich method. Here, the viral antigen is on the nasopharyngeal swab sample. The swab is placed into the testing device. The antigen flows from the swab on a strip of cellulose membrane, with an antibody labeled with colloidal gold at low speed.
Once the capture antibody bound on the cellulose membrane binds to the antigen-labeled antibody complex, a band of color appears on the strip. This test is typically offered on a simple lateral flow immunochromatography assay (LFIA) platform.
The identification of the test as positive is subject to visual error, besides which it is relatively insensitive and generates a higher proportion of false positives.
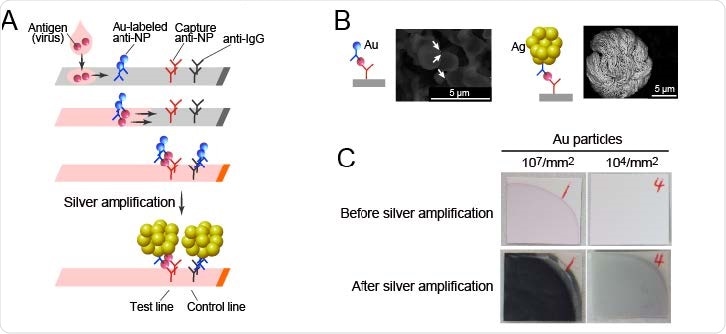
SARS-CoV-2 Ag-RDT with silver amplification technology. (A) Schematic diagram of lateral flow immunoassay with silver amplification technology. The antigen in the sample dropped into the device flows on the cellulose membrane together with the colloidal gold-labeled anti-SARS-CoV-2 NP antibody, and when captured by the membrane-immobilized capture antibody, it develops color and appears as a single band. Adherence of silver ions to the surface of a catalytic gold nanoparticle causes electrons to reduce the silver atoms, leading to the size enhancement followed by 1000-fold improvement in visibility. (B, C) Differences in SEM images (B) and naked eye visualized bands (C) with and without silver amplification.
Better antibodies
The current research reports using better antibodies to provide a solution to these issues of insensitivity and low positive predictive value. These monoclonal antibodies are highly specific and target the SARS-CoV-2 nucleocapsid protein (NP), but not that of closely related coronaviruses.
These antibodies form the basis of a very specific and sensitive LFIA. They bind strongly to distinct epitopes located at a distance from each other on the NP antigen's surface and do not interfere with each other.
In order to assess the potential for escape mutations to disrupt this test, they analyzed over 61,000 sequences of the viral NP. This revealed over a thousand different mutations at the nucleotide level. The two most common were found in 68% each. However, none of these mutations occur at the binding site for the NP antigen.
The use of the highly specific monoclonal antibodies results in a lack of cross-reactivity to other human coronaviruses and respiratory viruses, even the NP of SARS-CoV, which shares the most significant homology with SARS-CoV-2. On the other hand, it can detect wild-type SARS-CoV-2 in cultured cells, with a lower limit of detection relative to other current assays.
Silver amplification
With this test, the color band formed in the antigen's presence is enhanced by silver halide photography. Silver ions adhere to the surface of gold nanoparticles, of about 0.05 μm in diameter, and are reduced to metallic silver by electrons from the solution.
The metallic silver attaches firmly to the nanoparticles' surface, and the process is repeated over and over again within 30 seconds. The result is the generation of large particles, about 5-10 μm in diameter. This allows the visual output signal to be enhanced a thousand-fold. The researchers called this test the YCU-FF LFIA.
When tested on nasopharyngeal swabs from patients already diagnosed with COVID-19 by PCR, they found 82% positive percent agreement (PPA) between the two tests, the YCU-FF LFIA and the RT-PCR. When compared with other tests, the researchers observed that other Ag-RDTs failed to show false positives missed by this test and had a lower PPA.
This test performed better than other Ag-RDTs in samples with only moderate cycle threshold (Ct) values of 27-31.
What are the implications?
This test thus couples the specificity of the monoclonal antibodies with silver amplification, making it able to return a positive result with as few as 56 copies/μl of SARS-CoV-2 RNA in test specimens, allowing POC rapid testing with higher sensitivity and greater accuracy in both field testing centers and healthcare centers.
The YCU-FF LFIA can also identify all the variants of the virus in current circulation since none of the NP mutations affect the binding to either of these monoclonal antibodies.
The Ag-RDTs all tested well when the samples had high viral loads, corresponding to Ct<25, or 106 copies of viral RNA/mL. However, the YCU-FF LFIA was able to detect samples even when the viral load was only moderate, but not when the Ct was above 31, indicating a low viral titer.
The reasons could be either a below-threshold amount of viral antigen or the persistence of non-infectious viral RNA. If the latter is true, it could mean that a negative Ag-RDT indicates a non-infectious convalescent from an individual with acute infection, capable of transmitting the virus. This cannot be done using NAAT, and indicates an incredibly beneficial potential role for YCU-FF LFIA in identifying those who are most likely to spread the virus.
"The main advantages of Ag-RDTs are rapidity, ease of use, simplicity of interpretation, no need for technical capabilities or special infrastructure and decentralized POCT capability." These tests have so far been limited by their low sensitivity because of the high possibility of visual errors, leading to false negatives. The YCU-FF LFIA meets this need, making it a potentially valuable option as a supplementary test for NAATs in the diagnosis of COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Miyakawa, K. et al. (2021). SARS-CoV-2 antigen rapid diagnostic test enhanced with silver amplification technology. medRxiv preprint. doi: https://doi.org/10.1101/2021.01.27.21250659. https://www.medrxiv.org/content/10.1101/2021.01.27.21250659v1
- Peer reviewed and published scientific report.
Obata, Kazuaki, Kei Miyakawa, Toshiki Takei, Atsuhiko Wada, Yasuyoshi Hatayama, Hideaki Kato, Yayoi Kimura, Hisakuni Sekino, Junichi Katada, and Akihide Ryo. 2022. “Prospective Clinical Evaluation of the Diagnostic Accuracy of a Highly Sensitive Rapid Antigen Test Using Silver Amplification Technology for Emerging SARS-CoV-2 Variants.” Biomedicines 10 (11): 2801. https://doi.org/10.3390/biomedicines10112801. https://www.mdpi.com/2227-9059/10/11/2801.