In a study evaluating immune responses to the Pfizer-BioNTech vaccine, Ramin Herati and colleagues observed robust humoral (antibody) responses following two vaccine doses among individuals without previous SARS-CoV-2 infection (SARS-CoV-2-naïve).
However, SARS-CoV-2-experienced participants had a robust humoral response to the first dose but a muted response to the second dose.
In fact, SARS-CoV-2-experienced individuals had lower circulating levels of antigen-specific antibody-secreting cells (ASCs) following the second dose than they did following the first dose.
“These data highlight an important gap in our knowledge and may have major implications for how these vaccines should be used to prevent COVID-19,” writes Herati and the team.
A pre-print version of the research paper is available on the medRxiv* server while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
SARS-CoV-2 infection triggers important antibody responses
The SARS-COV-2 infection process is mediated by a surface structure called the spike protein, which binds to the host cell receptor angiotensin-converting enzyme 2 (ACE2) via its receptor-binding domain (RBD).
The spike protein comprises two subunits. Subunit 1 (S1) contains the RBD, which attaches to ACE2 via a receptor-binding motif (RBM) and subunit 2 (S2) enables the viral membrane to fuse with the host cell.
Following natural infection, antibodies targeting the spike RBD are thought to account for more than 90% of the neutralizing activity against SARS-CoV-2.
Research has shown that pre-existing immunity to SARS-CoV-2 is associated with protection against reinfection in humans and animals.
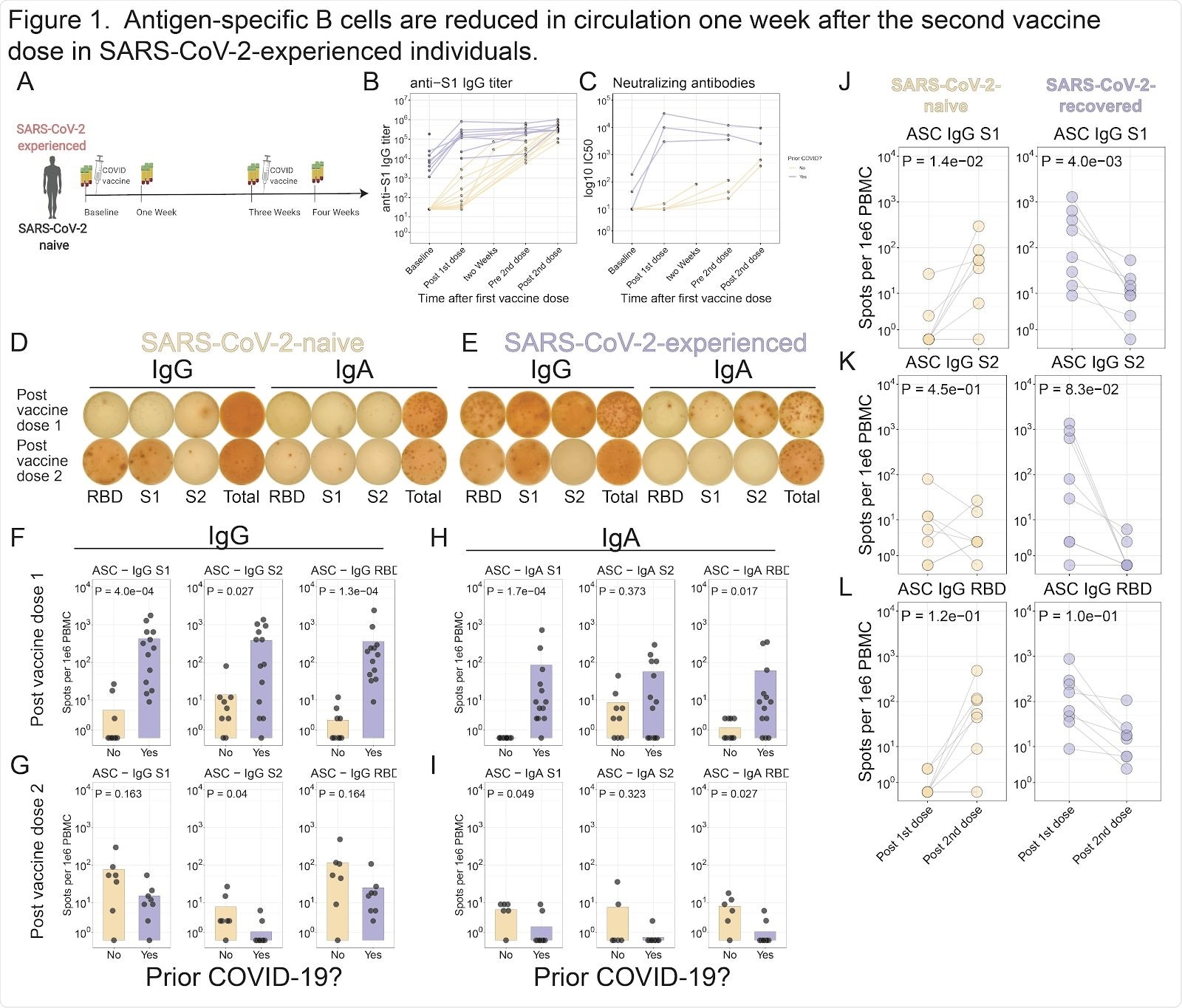
Antigen-specific B cells are reduced in circulation one week after the second vaccine dose in SARS-CoV-2-experienced individuals. A . Study schematic. B . S1 antibody titers were assessed for SARS-CoV-2 experienced (purple) and SARS-CoV-2-naive (yellow) subjects. Connected lines indicate repeated measurements of the same subjects. C . Neutralizing antibody titers were assessed using in vitro neutralization assay. D-E . ELISpot assays shown for a representative SARS-CoV-2-naive ( D ) or SARS-CoV-2-experienced ( E ) subject one week after each dose of vaccine. F-H . Summary statistics shown for ELISpot assays. For each panel, S1 (left), S2 (middle), or RBD (right) antigens are shown for IgG one week after first dose ( F ) or second dose ( G ), or for IgA one week after first dose ( H ) or second dose ( I ). J-L . ELISpot data shown for SARS-CoV-2-naive (left) or SARS-CoV-2-experienced (right) subjects for S1 ( J ), S2 ( K ), or RBD ( L ). Connected lines indicate repeated measurements from the same subjects.
Recently approved vaccines are based on the spike protein
Both the Pfizer-BioNTech and Moderna vaccines that have recently been FDA-approved for emergency use against SARS-CoV-2 are mRNA-based vaccines that encode a region of the spike protein.
These novel mRNA vaccines have been considered critical to ending the COVID-19 pandemic since they were shown to induce robust humoral responses against SARS-CoV-2 and to be 94% effective at preventing disease in large-scale clinical trials.
However, efficacy trials excluded individuals with a prior diagnosis of COVID-19 and only focused on individuals without previous SARS-CoV-2 exposure.
“As a result, little is known about the immune responses in SARS-CoV-2-experienced individuals, a setting which is relevant to hundreds of millions of people worldwide,” says Herati and colleagues.
“Indeed, more studies are needed to fully understand the breadth and quality of the immune response to these vaccines.”
What did the researchers do?
The team evaluated immune responses among 32 individuals (aged 24 to 62 years) who received the Pfizer-BioNTech BNT162b2 vaccine as a two-dose regimen.
Thirteen of the participants (median age 41 years) had previously been infected with SARS-CoV-2 and the remaining 19 (median age 39 years) were SARS-CoV-2-naïve.
All participants had immune responses assessed at approximate intervals before and after each dose of the vaccine.
What did the study find?
Following the first vaccine dose, anti-S1 immunoglobulin G (IgG) titers increased 47-fold among the SARS-CoV-2-experienced individuals and 2.6-fold among the SARS-CoV-2-naïve participants.
However, following the second dose, anti-S1 IgG titers only increased 1.4-fold among the SARS-CoV-2-experienced participants, whereas they increased 13-fold among SARS-CoV-2-naïve individuals.
One week after the second dose, anti-S1 IgG titers were similar between the two groups.
“These data demonstrate rapid and robust humoral responses after vaccination in both cohorts, but there was a minimal further increase in SARS-CoV-2-experienced subjects after the second vaccine dose,” says the team.
Similarly, while antigen-specific ASC responses increased following each vaccine dose among the SARS-CoV-2-naïve subjects, fewer circulating antigen-specific ASCs were detected among SARS-CoV-2-experienced participants after the second vaccine, compared with after the first dose.
After the second dose, reductions in circulating antigen-specific ASCs were observed for S1, S2 and the RBD among SARS-CoV-2-experienced participants.
What are the implications of the study?
“Here, we observed robust antibody responses in individuals who were SARS-CoV-2-naive,” says Herati and colleagues.
“In contrast, SARS-CoV-2-experienced subjects responded strongly to the first dose of vaccine with increased antibodies and antigen-specific ASC responses, but did not respond as strongly following a second vaccine dose,” they write.
The researchers say the findings highlight the need to investigate further the impact that prior immunological experience of SARS-CoV-2 may have on responses to COVID-19 vaccines.
“Future studies may be needed to determine whether more optimal dosing regimens are needed for durable, protective immunity to infection by the SARS-CoV-2 virus,” they conclude.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Herati RS, et al. Poor antigen-specific responses to the second BNT162b2 mRNA vaccine dose in SARS-CoV-2-experienced individuals. medRxiv, 2021. doi: https://doi.org/10.1101/2021.02.07.21251311, https://www.medrxiv.org/content/10.1101/2021.02.07.21251311v1
- Peer reviewed and published scientific report.
Samanovic, Marie I., Amber R. Cornelius, Sophie L. Gray-Gaillard, Joseph Richard Allen, Trishala Karmacharya, Jimmy P. Wilson, Sara Wesley Hyman, et al. 2022. “Robust Immune Responses Are Observed after One Dose of BNT162b2 MRNA Vaccine Dose in SARS-CoV-2–Experienced Individuals.” Science Translational Medicine 14 (631). https://doi.org/10.1126/scitranslmed.abi8961. https://www.science.org/doi/10.1126/scitranslmed.abi8961.